Abstract
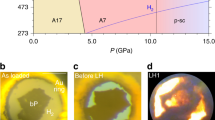
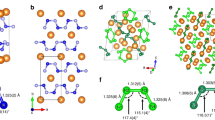
Polynitrogen molecules are attractive for high-energy-density materials due to energy stored in nitrogen–nitrogen bonds; however, it remains challenging to find energy-efficient synthetic routes and stabilization mechanisms for these compounds. Direct synthesis from molecular dinitrogen requires overcoming large activation barriers and the reaction products are prone to inherent inhomogeneity. Here we report the synthesis of planar N62− hexazine dianions, stabilized in K2N6, from potassium azide (KN3) on laser heating in a diamond anvil cell at pressures above 45 GPa. The resulting K2N6, which exhibits a metallic lustre, remains metastable down to 20 GPa. Synchrotron X-ray diffraction and Raman spectroscopy were used to identify this material, through good agreement with the theoretically predicted structural, vibrational and electronic properties for K2N6. The N62− rings characterized here are likely to be present in other high-energy-density materials stabilized by pressure. Under 30 GPa, an unusual N20.75−-containing compound with the formula K3(N2)4 was formed instead.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available at https://doi.org/10.6084/m9.figshare.19236573 (ref. 95), https://doi.org/10.6084/m9.figshare.19236609 (ref. 96) and https://doi.org/10.6084/m9.figshare.19228086.v1 (ref. 97). CSD 2022907 and 2094084 contain crystallographic data for the K3(N2)4 and K2N6 structures. These data can be obtained free of charge from FIZ Karlsruhe at www.ccdc.cam.ac.uk/structures. Source Data are provided with this paper.
References
Christe, K. O., Wilson, W. W., Sheehy, J. A. & Boatz, J. A. N5+: a novel homoleptic polynitrogen ion as a high energy density material. Angew. Chem. Int. Ed. 38, 2004–2009 (1999).
Lauderdale, W. J., Stanton, J. F. & Bartlett, R. J. Stability and energetics of metastable molecules: tetraazatetrahedrane (N4), hexaazabenzene (N6), and octaazacubane (N8). J. Phys. Chem. 96, 1173–1178 (1992).
Glukhovtsev, M. N., Jiao, H. & Schleyer, P. V. R. Besides N2, what is the most stable molecule composed only of nitrogen atoms? Inorganic Chemistry 35, 7124–7133 (1996).
Hirshberg, B., Gerber, R. B. & Krylov, A. I. Calculations predict a stable molecular crystal of N8. Nat Chem 6, 52–56 (2014).
Zahariev, F., Hooper, J., Alavi, S., Zhang, F. & Woo, T. K. Low-pressure metastable phase of single-bonded polymeric nitrogen from a helical structure motif and first-principles calculations. Phys. Rev. B 75, 140101 (2007).
Mailhiot, C., Yang, L. H. & McMahan, A. K. Polymeric nitrogen. Phys. Rev. B 46, 14419 (1992).
Tobita, M. & Bartlett, R. J. Structure and stability of N6 isomers and their spectroscopic characteristics. J. Phys. Chem. A 105, 4107–4113 (2001).
Samartzis, P. C. & Wodtke, A. M. All-nitrogen chemistry: how far are we from N60? Int. Rev. Phys. Chem. 25, 527 (2006).
Samartzis, P. C. & Wodtke, A. M. Casting a new light on azide photochemistry: photolytic production of cyclic-N3. Phys. Chem. Chem. Phys. 9, 3054–3066 (2007).
Eremets, M. I., Gavriliuk, A. G., Trojan, I. A., Dzivenko, D. A. & Boehler, R. Single-bonded cubic form of nitrogen. Nat. Mater. 3, 558–563 (2004).
Wilson, K. J., Perera, S. A., Bartlett, R. J. & Watts, J. D. Stabilization of the pseudo-benzene N6 ring with oxygen. J. Phys. Chem. A 105, 7693–7699 (2001).
Wang, W. et al. High-pressure bonding mechanism of selenium nitrides. Inorganic Chemistry 58, 2397–2402 (2019).
Zhang, S., Zhao, Z., Liu, L. & Yang, G. Pressure-induced stable BeN4 as a high-energy density material. J. Power Sources 365, 155–1161 (2017).
Liu, Z. et al. Nitrogen-rich GaN5 and GaN6 as high energy density materials with modest synthesis condition. Phys. Lett. A 383, 125859 (2019).
Li, J., Sun, L., Wang, X., Zhu, H. & Miao, M. Simple route to metal cyclo-N5− salt: high-pressure synthesis of CuN5. J. Phys. Chem. C 122, 22339–22344 (2018).
Zhang, Y. et al. Diverse ruthenium nitrides stabilized under pressure: a theoretical prediction. Sci. Rep. 6, 33506 (2016).
Straka, M. N6 ring as a planar hexagonal ligand in novel M(η6-N6) species. Chem. Phys. Lett. 358, 531–536 (2002).
Zhang, J., Oganov, A. R., Li, X. & Niu, H. Pressure-stabilized hafnium nitrides and their properties. Phys. Rev. B 95, 020103 (2017).
Kvashnin, A. G., Oganov, A. R., Samtsevich, A. I. & Allahyari, Z. Computational Search for novel hard chromium-based materials. J. Phys. Chem. Lett. 8, 755–764 (2017).
Yu, S. et al. Emergence of novel polynitrogen molecule-like species, covalent chains, and layers in magnesium–nitrogen MgxNy phases under high pressure. J. Phys. Chem. C 121, 11037–11046 (2017).
Huang, B. & Frapper, G. Barium–nitrogen phases under pressure: emergence of structural diversity and nitrogen-rich compounds. Chem. Mater. 30, 7623–7636 (2018).
Zhang, C., Sun, C., Hu, B., Yu, C. & Lu, M. Synthesis and characterization of the pentazolate anion cyclo-N5- in (N5)6(H3O)3(NH4)4Cl. Science 355, 374–376 (2017).
Xu, Y. et al. A series of energetic metal pentazolate hydrates. Nature 549, 78 (2017).
Xu, Y., Lin, Q., Wang, P. & Lu, M. Syntheses, crystal structures and properties of a series of 3D metal–inorganic frameworks containing pentazolate anion. Chem. Asian J. 13, 1669–1673 (2018).
Sun, C. et al. Synthesis of AgN5 and its extended 3D energetic framework. Nat. Commun. 9, 1269 (2018).
Zhang, W. et al. Stabilization of the pentazolate anion in a zeolitic architecture with Na20N60 and Na24N60 nanocages. Angew. Chem. Int. Ed. 57, 2592–2595 (2018).
Yao, Y., Lin, Q., Zhou, X. & Lu, M. Recent research on the synthesis pentazolate anion cyclo-N5−. FirePhysChem 1, 33–45 (2021).
Christe, K. O. Polynitrogen chemistry enters the ring. Science 355, 351–351 (2017).
Wozniak, D. R. & Piercey, D. G. Review of the current synthesis and properties of energetic pentazolate and derivatives thereof. Engineering 6, 981–991 (2020).
Ren, G. et al. Theoretical perspective on the reaction mechanism from arylpentazenes to arylpentazoles: new insights into the enhancement of cyclo-N5 production. Chem. Commun. 55, 2628–2631 (2019).
Bykov, M. et al. Fe-N system at high pressure reveals a compound featuring polymeric nitrogen chains. Nat Commun 9, 2756 (2018).
Laniel, D. et al. Synthesis of magnesium-nitrogen salts of polynitrogen anions. Nat. Commun. 10, 7 (2019).
Bykov, M. et al. High-pressure synthesis of Dirac materials: layered van der Waals bonded BeN4 polymorph. Phys. Rev. Lett. 126, 175501 (2021).
Bykov, M. et al. Stabilization of polynitrogen anions in tantalum–nitrogen compounds at high pressure. Angew. Chem. Int. Ed. 60, 9003–9008 (2021).
Bykov, M. et al. High-pressure synthesis of a nitrogen-rich inclusion compound ReN8⋅xN2 with conjugated polymeric nitrogen chains. Angew. Chem. Int. Ed. 57, 9048–9053 (2018).
Bykov, M. et al. High-pressure synthesis of metal–inorganic frameworks Hf4N20⋅N2, WN8⋅N2, and Os5N28⋅3N2 with polymeric nitrogen linkers. Angew. Chem. Int. Ed. 59, 10321–10326 (2020).
Salke, N. P. et al. Tungsten hexanitride with single-bonded armchairlike hexazine structure at high pressure. Phys. Rev. Lett. 126, 065702 (2021).
Wang, X., Li, J., Zhu, H., Chen, L. & Lin, H. Polymerization of nitrogen in cesium azide under modest pressure. J. Chem. Phys. 141, 044717 (2014).
Peng, F., Yao, Y., Liu, H. & Ma, Y. Crystalline LiN5 predicted from first-principles as a possible high-energy material. J. Phys. Chem. Lett. 6, 2363–2366 (2015).
Shen, Y. et al. Novel lithium—nitrogen compounds at ambient and high pressures. Sci. Rep. 5, 14204 (2015).
Steele, B. A. & Oleynik, I. I. Sodium pentazolate: a nitrogen rich high energy density material. Chem. Phys. Lett. 643, 21–26 (2016).
Steele, B. A. & Oleynik, I. I. Novel potassium polynitrides at high pressures. J. Phys. Chem. A 121, 8955–8961 (2017).
Xia, K. et al. Pressure-stabilized high-energy-density alkaline-earth-metal pentazolate salts. J. Phys. Chem. C 123, 10205–10211 (2019).
Zhang, J., Zeng, Z., Lin, H.-Q. & Li, Y.-L. Pressure-induced planar N6 rings in potassium azide. Sci. Rep. 4, 4358 (2014).
Steele, B. A. et al. High-pressure synthesis of a pentazolate salt. Chem. Mater. 29, 735–741 (2016).
Prasad, D. L. V. K., Ashcroft, N. W. & Hoffmann, R. Evolving structural diversity and metallicity in compressed lithium azide. J. Phys. Chem. C 117, 20838–20846 (2013).
Li, J. et al. Pressure-induced polymerization of nitrogen in potassium azides. EPL https://doi.org/10.1209/0295-5075/104/16005 (2013).
Wang, X. et al. Polymerization of nitrogen in lithium azide. J. Chem. Phys. https://doi.org/10.1063/1.4826636 (2013).
Zhang, M., Yan, H., Wei, Q., Wang, H. & Wu, Z. Novel high-pressure phase with pseudo-benzene “N6” molecule of LiN3. EPL https://doi.org/10.1209/0295-5075/101/26004 (2013).
Zhang, M. et al. Structural and electronic properties of sodium azide at high pressure: a first principles study. Solid State Commun. 161, 13–18 (2013).
Yu, H. et al. Polymerization of nitrogen in ammonium azide at high pressures. J. Phys. Chem. C 119, 25268–25272 (2015).
Zhang, M., Yan, H., Wei, Q. & Liu, H. A new high-pressure polymeric nitrogen phase in potassium azide. RSC Adv. 5, 11825–11830 (2015).
Laniel, D., Weck, G., Gaiffe, G., Garbarino, G. & Loubeyre, P. High-pressure synthesized lithium pentazolate compound metastable under ambient conditions. J. Phys. Chem. Lett. 9, 1600–1604 (2018).
Laniel, D., Weck, G. & Loubeyre, P. Direct reaction of nitrogen and lithium up to 75 GPa: synthesis of the Li3N, LiN, LiN2, and LiN5 compounds. Inorg. Chem. 57, 10685–10693 (2018).
Bykov, M. et al. Stabilization of pentazolate anions in the high-pressure compounds Na2N5 and NaN5 and in the sodium pentazolate framework NaN5·N2. Dalton Trans. 50, 7229–7237 (2021).
Eremets, M. I. et al. Polymerization of nitrogen in sodium azide. J. Chem. Phys. 120, 10618–10623 (2004).
Medvedev, S. A. et al. Phase stability of lithium azide at pressures up to 60 GPa. J. Phys. Condens. Matter https://doi.org/10.1088/0953-8984/21/19/195404 (2009).
Zhu, H. et al. Pressure-induced series of phase transitions in sodium azide. J. Appl. Phys. https://doi.org/10.1063/1.4776235 (2013).
Ji, C. et al. Pressure-induced phase transition in potassium azide up to 55 GPa. J. Appl. Phys. 111, 112613 (2012).
Ji, C. et al. High pressure X-ray diffraction study of potassium azide. J. Phys. Chem. Solids 72, 736–739 (2011).
Holtgrewe, N., Lobanov, S. S., Mahmood, M. F. & Goncharov, A. F. Photochemistry within compressed sodium azide. J. Phys. Chem. C 120, 28176–28185 (2016).
Bykov, M. et al. Dinitrogen as a universal electron acceptor in solid-state chemistry: an example of uncommon metallic compounds Na3(N2)4 and NaN2. Inorg. Chem. 59, 14819–14826 (2020).
Hou, D. et al. Phase transition and structure of silver azide at high pressure. J. Appl. Phys. 110, 023524 (2011).
Tomasino, D., Kim, M., Smith, J. & Yoo, C.-S. Pressure-induced symmetry-lowering transition in dense nitrogen to layered polymeric nitrogen (LP-N) with colossal Raman intensity. Phys. Rev. Lett. 113, 205502 (2014).
Olijnyk, H. High pressure X-ray diffraction studies on solid N2 up to 43.9 GPa. J. Chem. Phys. 93, 8968–8972 (1990).
Kantor, I. et al. BX90: a new diamond anvil cell design for X-ray diffraction and optical measurements. Rev. Sci. Instrum. 83, 125102 (2012).
Boehler, R. New diamond cell for single-crystal X-ray diffraction. Rev. Sci. Instrum. 77, 115103 (2006).
Prakapenka, V. B. et al. Advanced flat top laser heating system for high pressure research at GSECARS: application to the melting behavior of germanium. High Pressure Res. 28, 225–235 (2008).
Cheng, P. et al. Polymorphism of polymeric nitrogen at high pressures. J. Chem. Phys. 152, 244502 (2020).
Goncharov, A. F. et al. Backbone NxH compounds at high pressures. J. Chem. Phys. https://doi.org/10.1063/1.4922051 (2015).
Holtgrewe, N., Greenberg, E., Prescher, C., Prakapenka, V. B. & Goncharov, A. F. Advanced integrated optical spectroscopy system for diamond anvil cell studies at GSECARS. High Pressure Res. 39, 457–470 (2019).
Sheldrick, G. M. SHELXT— integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Prescher, C. & Prakapenka, V. B. DIOPTAS: a program for reduction of two-dimensional X-ray diffraction data and data exploration. High Pressure Res. 35, 223–230 (2015).
Václav, P., Michal, D. & Lukáš, P. Crystallographic computing system JANA2006: general features. Zeit. Kristallogr. 229, 345–352 (2014).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Togo, A. & Tanaka, I. First principles phonon calculations in materials science. Scr. Mater. 108, 1 (2015).
Ganose, A. M., Jackson, A. J. & Scanlton, D. O. SUMO: command-line tools for plotting and analysis of periodic ab initio calculations. J. Open Source Softw. 3, 717 (2018).
Therrien, F., Graf, P. & Stevanović, V. Matching crystal structures atom-to-atom. J. Chem. Phys. 152, 074106 (2020).
Stevanović, V. et al. Predicting kinetics of polymorphic transformations from structure mapping and coordination analysis. Phys. Rev. Mater. 2, 033802 (2018).
Hart, G. L. W. & Forcade, R. W. Algorithm for generating derivative structures. Phys. Rev. B 77, 224115 (2008).
Qian, G.-R. et al. Variable cell nudged elastic band method for studying solid–solid structural phase transitions. Comput. Phys. Commun. 184, 2111–2118 (2013).
Oganov, A. R. & Glass, C. W. Crystal structure prediction using ab initio evolutionary techniques: principles and applications. J. Chem. Phys. https://doi.org/10.1063/1.2210932 (2006).
Oganov, A. R., Lyakhov, A. O. & Valle, M. How evolutionary crystal structure prediction works—and why. Acc. Chem. Res. 44, 227–237 (2011).
Oganov, A. R., Ma, Y., Lyakhov, A. O., Valle, M. & Gatti, C. Evolutionary crystal structure prediction as a method for the discovery of minerals and materials. Rev. Mineralogy Geochem. 71, 271–298 (2010).
Henkelman, G., Uberuaga, B. P. & Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Monkhorst, H. J. & Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 13, 5188–5192 (1976).
Samtsevich, A. Details of Theoretical Calculations of the Transition Pathways Between the Candidate Structures (FigShare, 2022); https://doi.org/10.6084/m9.figshare.19236573
Chepkasov, I. Details of Theoretical Calculations of the Electronic and Phonon Band Structure of Novel High-Pressure K2N6 (FigShare, 2022); https://doi.org/10.6084/m9.figshare.19236609
Bykov, M. Synchrotron Dataset of laser-heated KN3 at ~50 GPa (FigShare, 2022); https://doi.org/10.6084/m9.figshare.19228086.v1
Acknowledgements
This work at ISSP was supported by the National Natural Science Foundation of China (grant nos. 11504382, 21473211, 11674330, 51672279, 11874361, 11774354 and 51727806), the CASHIPS Director’s Fund (grant no. YZJJ201705), the Chinese Academy of Science (grant nos. YZ201524 and YZJJ2020QN22) and a Science Challenge Project (no. TZ201601). This research at Carnegie and APS was sponsored by the Army Research Office and was accomplished under the Cooperative Agreement Number W911NF-19-2-0172. A.R.O., I.C. and A.I.S acknowledge funding from Russian Science Foundation (grant no. 19-72-30043). GeoSoilEnviroCARS (The University of Chicago, Sector 13), Advanced Photon Source (APS), Argonne National Laboratory is supported by the National Science Foundation—Earth Sciences (EAR-1634415). Use of the GSECARS Raman System was supported by the NSF MRI Proposal (EAR-1531583). The Advanced Photon Source is a US Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract no. DE-AC02-06CH11357. The theoretical calculations were performed using Arkuda and Oleg supercomputer of Skoltech.
Author information
Authors and Affiliations
Contributions
Y.W., M.B. and A.F.G. conceived the research and designed the experiments. Y.W., M.B., X.Z., E.G., S.C., V.B.P. and S.-q.J performed the experiment. E.B., Y.W., M.B. and A.F.G. analysed the data. I.C., A.S. and A.R.O. performed and analysed the calculations. A.F.G., Y.W., M.B. and A.S. wrote the manuscript. All authors reviewed and discussed the manuscript during preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Karl Christe, Thomas Klapötke and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–11, Tables 1–5 and an ORTEP illustration of the crystal structures.
Supplementary Data 1
Crystallographic structure file for K2N6 (CCDC 2094084).
Supplementary Data 2
Crystallographic structure file for K3(N2)4 (CCDC 2022907).
Supplementary Data 3
Source data for Supplementary Figs. 5 and 6a,b, and uncompressed ascii data.
Supplementary Data 4
Source data for Supplementary Fig. 9 and uncompressed ascii data.
Supplementary Data 5
Source data for Supplementary Fig. 11 and ascii data.
Source data
Source Data Fig. 3
Uncompressed ascii data.
Source Data Fig. 4
Uncompressed ascii data.
Rights and permissions
About this article
Cite this article
Wang, Y., Bykov, M., Chepkasov, I. et al. Stabilization of hexazine rings in potassium polynitride at high pressure. Nat. Chem. 14, 794–800 (2022). https://doi.org/10.1038/s41557-022-00925-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00925-0
This article is cited by
-
Stabilization of N6 and N8 anionic units and 2D polynitrogen layers in high-pressure scandium polynitrides
Nature Communications (2024)
-
Benzene-like N6 hexazine rings
Nature Chemistry (2023)
-
Aromatic hexazine [N6]4− anion featured in the complex structure of the high-pressure potassium nitrogen compound K9N56
Nature Chemistry (2023)
-
Materials under extreme conditions using large X-ray facilities
Nature Reviews Methods Primers (2023)