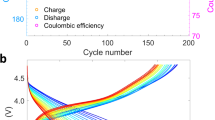
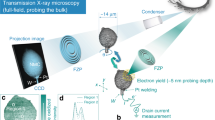
Abstract
Nickel-rich layered oxides are envisaged as key near-future cathode materials for high-energy lithium-ion batteries. However, their practical application has been hindered by their inferior cycle stability, which originates from chemo-mechanical failures. Here we probe the solid-state synthesis of LiNi0.6Co0.2Mn0.2O2 in real time to better understand the structural and/or morphological changes during phase evolution. Multi-length-scale observations—using aberration-corrected transmission electron microscopy, in situ heating transmission electron microscopy and in situ X-ray diffraction—reveal that the overall synthesis is governed by the kinetic competition between the intrinsic thermal decomposition of the precursor at the core and the topotactic lithiation near the interface, which results in spatially heterogeneous intermediates. The thermal decomposition leads to the formation of intergranular voids and intragranular nanopores that are detrimental to cycling stability. Furthermore, we demonstrate that promoting topotactic lithiation during synthesis can mitigate the generation of defective structures and effectively suppress the chemo-mechanical failures.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data supporting the findings of this study are available within the article and its Supplementary Information and also from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
The code for calculation of particle average TEM intensity is available at https://github.com/parkhayoungg/Parklab_nchem_NCM_code.
References
Liu, Z., Yu, A. & Lee, J. Y. Synthesis and characterization of LiNi1−x−yCoxMnyO2 as the cathode materials of secondary lithium batteries. J. Power Sources 81–82, 416–419 (1999).
Noh, H.-J., Youn, S., Yoon, C. S. & Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 233, 121–130 (2013).
Sharma, S. S. & Manthiram, A. Towards more environmentally and socially responsible batteries. Energy Environ. Sci. 13, 4087–4097 (2020).
Kim, J. et al. Prospect and reality of Ni-rich cathode for commercialization. Adv. Energy Mater. 8, 1702028 (2018).
Li, W., Erickson, E. M. & Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 5, 26–34 (2020).
de Biasi, L. et al. Chemical, structural, and electronic aspects of formation and degradation behavior on different length scales of Ni-rich NCM and Li-rich HE-NCM cathode materials in Li-ion batteries. Adv. Mater. 31, 1900985 (2019).
Jung, S.-K. et al. Understanding the degradation mechanisms of LiNi0.5Co0.2Mn0.3O2 cathode material in lithium ion batteries. Adv. Energy Mater. 4, 1300787 (2014).
Yan, P., Zheng, J., Zhang, J.-G. & Wang, C. Atomic resolution structural and chemical imaging revealing the sequential migration of Ni, Co, and Mn upon the battery cycling of layered cathode. Nano Lett. 17, 3946–3951 (2017).
Zheng, H., Sun, Q., Liu, G., Song, X. & Battaglia, V. S. Correlation between dissolution behavior and electrochemical cycling performance for LiNi1/3Co1/3Mn1/3O2-based cells. J. Power Sources 207, 134–140 (2012).
Kim, J. et al. A highly stabilized nickel-rich cathode material by nanoscale epitaxy control for high-energy lithium-ion batteries. Energy Environ. Sci. 11, 1449–1459 (2018).
Jung, R., Metzger, M., Maglia, F., Stinner, C. & Gasteiger, H. A. Oxygen release and its effect on the cycling stability of LiNixMnyCozO2 (NMC) cathode materials for Li-ion batteries. J. Electrochem. Soc. 164, A1361–A1377 (2017).
Mu, L. et al. Oxygen release induced chemomechanical breakdown of layered cathode materials. Nano Lett. 18, 3241–3249 (2018).
Li, S. et al. Mutual modulation between surface chemistry and bulk microstructure within secondary particles of nickel-rich layered oxides. Nat. Commun. 11, 4433 (2020).
Li, W., Asl, H. Y., Xie, Q. & Manthiram, A. Collapse of LiNi1–x–yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 141, 5097–5101 (2019).
Romano Brandt, L. et al. Synchrotron X-ray quantitative evaluation of transient deformation and damage phenomena in a single nickel-rich cathode particle. Energy Environ. Sci. 13, 3556–3566 (2020).
Xu, Z. et al. Charge distribution guided by grain crystallographic orientations in polycrystalline battery materials. Nat. Commun. 11, 83 (2020).
Tian, C. et al. Charge heterogeneity and surface chemistry in polycrystalline cathode materials. Joule 2, 464–477 (2018).
Xiao, B. et al. Revealing the atomic origin of heterogeneous Li-ion diffusion by probing Na. Adv. Mater. 31, 1805889 (2019).
Heenan, T. M. M. et al. Resolving Li-ion battery electrode particles using rapid lab-based X-ray nano-computed tomography for high-throughput quantification. Adv. Sci. 7, 2000362 (2020).
Mao, Y. et al. High-voltage charging-induced strain, heterogeneity, and micro-cracks in secondary particles of a nickel-rich layered cathode material. Adv. Funct. Mater. 29, 1900247 (2019).
Lin, F. et al. Surface reconstruction and chemical evolution of stoichiometric layered cathode materials for lithium-ion batteries. Nat. Commun. 5, 3529 (2014).
Zhu, J. et al. Atomic-level understanding of surface reconstruction based on Li[NixMnyCo1–x–y]O2 single-crystal studies. ACS Appl. Energy Mater. 3, 4799–4811 (2020).
Duan, Y. et al. Insights into Li/Ni ordering and surface reconstruction during synthesis of Ni-rich layered oxides. J. Mater. Chem. A 7, 513–519 (2019).
Zhang, H. et al. Facet-dependent rock-salt reconstruction on the surface of layered oxide cathodes. Chem. Mater. 30, 692–699 (2018).
Zhang, M.-J. et al. Cooling induced surface reconstruction during synthesis of high-Ni layered oxides. Adv. Energy Mater. 9, 1901915 (2019).
Ahmed, S. et al. The role of intragranular nanopores in capacity fade of nickel-rich layered Li(Ni1–x–yCoxMny)O2 cathode materials. ACS Nano 13, 10694–10704 (2019).
Pokle, A. et al. In situ monitoring of thermally induced effects in nickel-rich layered oxide cathode materials at the atomic level. ACS Appl. Mater. Interfaces 12, 57047–57054 (2020).
Shukla, A. K. et al. Unravelling structural ambiguities in lithium- and manganese-rich transition metal oxides. Nat. Commun. 6, 8711 (2015).
Yan, P. et al. Ni and Co segregations on selective surface facets and rational design of layered lithium transition-metal oxide cathodes. Adv. Energy Mater. 6, 1502455 (2016).
Li, L., Hu, J., Xiao, J. & Wang, C. Origin, nature, and the dynamic behavior of nanoscale vacancy clusters in Ni-rich layered oxide cathodes. ACS Appl. Mater. Interfaces 13, 18849–18855 (2021).
Lin, R. et al. Hierarchical nickel valence gradient stabilizes high-nickel content layered cathode materials. Nat. Commun. 12, 2350 (2021).
Qian, G. et al. Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 27, 140–149 (2020).
Zhang, F. et al. Surface regulation enables high stability of single-crystal lithium-ion cathodes at high voltage. Nat. Commun. 11, 3050 (2020).
Shim, J.-H. et al. Effects of heat-treatment atmosphere on electrochemical performances of Ni-rich mixed-metal oxide (LiNi0.80Co0.15Mn0.05O2) as a cathode material for lithium ion battery. Electrochim. Acta 138, 15–21 (2014).
Wang, D. et al. Synthetic control of kinetic reaction pathway and cationic ordering in high-Ni layered oxide cathodes. Adv. Mater. 29, 1606715 (2017).
Zhao, J. et al. In situ probing and synthetic control of cationic ordering in Ni-rich layered oxide cathodes. Adv. Energy Mater. 7, 1601266 (2017).
Zhang, M.-J. et al. Cationic ordering coupled to reconstruction of basic building units during synthesis of high-Ni layered oxides. J. Am. Chem. Soc. 140, 12484–12492 (2018).
Bai, J. et al. Kinetic pathways templated by low-temperature intermediates during solid-state synthesis of layered oxides. Chem. Mater. 32, 9906–9913 (2020).
Bianchini, M. et al. The interplay between thermodynamics and kinetics in the solid-state synthesis of layered oxides. Nat. Mater. 19, 1088–1095 (2020).
Hua, W. et al. Chemical and structural evolution during the synthesis of layered Li(Ni,Co,Mn)O2 oxides. Chem. Mater. 32, 4984–4997 (2020).
Wang, S. et al. Kinetic control of long-range cationic ordering in the synthesis of layered Ni-rich oxides. Adv. Funct. Mater. 31, 2009949 (2021).
Maier, J. Thermodynamics of electrochemical lithium storage. Angew. Chem. Int. Ed. 52, 4998–5026 (2013).
Li, Y. et al. Synthesis of full concentration gradient cathode studied by high energy X-ray diffraction. Nano Energy 19, 522–531 (2016).
Dong, B. et al. One-step synthesis of free-standing α-Ni(OH)2 nanosheets on reduced graphene oxide for high-performance supercapacitors. Nanotechnology 25, 435403 (2014).
Wang, D., Xu, R., Wang, X. & Li, Y. NiO nanorings and their unexpected catalytic property for CO oxidation. Nanotechnology 17, 979–983 (2006).
Abbas, S. A., Iqbal, M. I., Kim, S.-H., Abbas Khan, H. & Jung, K.-D. Facile synthesis of alfa-nickel hydroxide by an ultrasound-assisted method and its application in energy storage devices. Appl. Surf. Sci. 474, 218–226 (2019).
Liu, Q. et al. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 3, 936–943 (2018).
Seong, W. M. et al. Abnormal self-discharge in lithium-ion batteries. Energy Environ. Sci. 11, 970–978 (2018).
Zhang, J.-N. et al. Trace doping of multiple elements enables stable battery cycling of LiCoO2 at 4.6 V. Nat. Energy 4, 594–603 (2019).
Nam, G. W. et al. Capacity fading of Ni-rich NCA cathodes: effect of microcracking extent. ACS Energy Lett. 4, 2995–3001 (2019).
Liu, X. et al. Essential effect of the electrolyte on the mechanical and chemical degradation of LiNi0.8Co0.15Al0.05O2 cathodes upon long-term cycling. J. Mater. Chem. A 9, 2111–2119 (2021).
Xu, C. et al. Bulk fatigue induced by surface reconstruction in layered Ni-rich cathodes for Li-ion batteries. Nat. Mater. 20, 84–92 (2021).
Yan, P. et al. Tailoring grain boundary structures and chemistry of Ni-rich layered cathodes for enhanced cycle stability of lithium-ion batteries. Nat. Energy 3, 600–605 (2018).
Yoon, M. et al. Reactive boride infusion stabilizes Ni-rich cathodes for lithium-ion batteries. Nat. Energy 6, 362–371 (2021).
Zou, L. et al. Lattice doping regulated interfacial reactions in cathode for enhanced cycling stability. Nat. Commun. 10, 3447 (2019).
Jung, C.-H. et al. Revisiting the role of Zr doping in Ni-rich layered cathodes for lithium-ion batteries. J. Mater. Chem. A 9, 17415–17424 (2021).
Kim, U.-H. et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge. Nat. Energy 5, 860–869 (2020).
Jung, C.-H. et al. New insight into microstructure engineering of Ni-rich layered oxide cathode for high performance lithium ion batteries. Adv. Funct. Mater. 31, 2010095 (2021).
Song, S. H. et al. High-voltage-driven surface structuring and electrochemical stabilization of Ni-rich layered cathode materials for Li rechargeable batteries. Adv. Energy Mater. 10, 2000521 (2020).
Acknowledgements
This work was supported by the Institute of Basic Science (IBS-R006-A2 and IBS-R006-D1) and by the Defense Challengeable Future Technology Program of the Agency for Defense Development, Republic of Korea (contract no. UC190025RD); financially supported by SK Innovation; and supported by a National Research Foundation of Korea grant funded by the Korean government’s Ministry of Science and ICT (no. 2021R1C1C2012688). K.K. acknowledges support from Samsung SDI. Hayoung Park, S.K., J.K. and J.P. acknowledge National Research Foundation of Korea grants, funded by the Korean government’s Ministry of Science and ICT (no. NRF-2017R1A5A1015365, no. NRF-2021M3H4A1A02045962 and no. NRF-2020R1A2C2101871). Hayoung Park, Y.J. and J.P. acknowledge support by the Samsung Research Funding & Incubation Center of Samsung Electronics under project no. SRFC-MA2002-03 for the development of the in situ TEM method. K.S. acknowledges the work at the Korea Institute of Materials Science by the Fundamental Research Program of the Korea Institute of Materials Science (grant no. PNK8450).
Author information
Authors and Affiliations
Contributions
Hyeokjun Park, Hayoung Park, J.P. and K.K. conceived the original idea and designed the research project. Hyeokjun Park and Hayoung Park carried out the synthesis and the structural and electrochemical characterizations of the materials; participated in all experiments and relevant analyses; and led the project direction. K.S. conducted the in situ heating environment TEM imaging experiments. S.H.S. and H.K. performed the in situ heating X-ray diffraction measurements and provided constructive advice on the analysis of the diffraction results. S.K. conducted the focused ion beam preparation and the high-resolution TEM and high-resolution STEM imaging experiments. K.-H.K., D.E., Y.J., J.K. and W.M.S. offered valuable comments and discussion on the experimental design and analyses. Hyeokjun Park, Hayoung Park, J.P. and K.K. wrote the manuscript with the help of the other authors. The manuscript reflects the contributions of all authors. J.P. and K.K. supervised all aspects of the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Jianming Bai, Helmut Ehrenberg, Kerstin Volz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Ex situ XRD pattern of the mixture of transition-metal hydroxide and lithium hydroxide with heating.
The ex situ samples were collected at different temperatures following the heating profiles of the conventional synthesis of NCM622. Note that the TM(OH)2 is a CdI2-type layered structure in the \(P{\bar 3}m1\) space group whose main Bragg reflection appears near 19.5°.
Extended Data Fig. 2 In situ XRD pattern of the mixture of transition-metal hydroxide and lithium carbonate.
(a) In situ XRD pattern at given equilibrated temperature for calcinations of precursor for layered oxide. (b) Phase and structural evolution during the synthesis. The phase fraction of Li2CO3 might be overestimated at the certain temperature region near 250 °C because of the decrease in the overall crystallinity of mixed transition metal compounds that undergo the decomposition. Nevertheless, the phase analysis result clearly verifies the distinct synthetic mechanism with the usage of Li2CO3 by which thermal dehydration of NCM hydroxide precursor is preceded into the oxide phase, followed by synthetic reaction with Li2CO3 at a much higher temperature, resulting in slow formation of layered oxide structures.
Extended Data Fig. 3 Cross-sectional SEM and TEM images of transition-metal hydroxide mixture with lithium hydroxide heated ex situ at 500 °C.
(a) Cross-sectional SEM image. TEM images and SAED pattern of primary particles at the (b) core and (c) shell region, as marked in (a). The radial heterogeneity was alleviated to some extent, which agrees with the results of the negative intensity difference at higher temperature (> 340 °C) in Fig. 2f. The SAED patterns on the sample also indicate that both the core and surface regions of the particle evolved to the lithium-containing layered oxide. This result suggests that the continuous inward supply of lithium through the interface could induce the full lithiation within the secondary particle at this temperature, although the path toward the layered structure formation differs for the shell and core regions.
Extended Data Fig. 4 EELS line scan results of nanopore in transition-metal hydroxide heated at 500 °C.
(a) STEM image of nanopore within primary particle, (b) EELS Mn L-edge, Co L-edge, and Ni L-edge in corresponding area. The oxidation state of the transition metal in the nanopore was slightly more reduced, corresponding to the typical transition-metal states in the rock-salt phase.
Extended Data Fig. 5 TG analysis results for mixture of transition-metal hydroxide and lithium hydroxide.
(a) TG curve with different holding times at 200 °C to 6 h with a heating rate of 5 °C min−1. (b) Column graph showing the percentage of weight loss from lithiation and dehydration of transition-metal hydroxide as a function of holding time. Each weight loss is derived from the value measured at 200–225 °C (before 225 °C) and 225–300 °C (after 225 °C) for lithiation and dehydration, respectively. The temperature of 225 °C corresponds to the local minimum point between the two peaks in the TG curve. We set the maximum of the temperature range for thermal dehydration to 300 °C, beyond which negligible thermal activity is observed in the TG curve for the mixture of transition-metal hydroxide and lithium hydroxide. The inversely proportional relationship of the weight loss for the two events indicates that it is reasonable that as much synthetic lithiation as possible occurs. The amount of remaining transition-metal hydroxide precursor that can be thermally decomposed is reduced.
Extended Data Fig. 6 Electrochemical properties of NCM electrodes at different rates.
(a) Capacity retention at 1 C. (b) Rate capability and capacity retention at 0.1 C.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10, Tables 1 and 2, and Notes 1–4.
Supplementary Video 1
In situ TEM snapshots during heating of transition-metal hydroxide and lithium hydroxide from 150 °C to 350 °C.
Supplementary Data 1
Source data for the supplementary figures.
Source data
Source Data Fig. 1
Source data for Fig. 1.
Source Data Fig. 2
Source data for Fig. 2.
Source Data Fig. 4
Source data for Fig. 4.
Source Data Extended Data Fig. 1
Source data for Extended Data Fig. 1.
Source Data Extended Data Fig. 2
Source data for Extended Data Fig. 2.
Source Data Extended Data Fig. 4
Source data for Extended Data Fig. 4.
Source Data Extended Data Fig. 5
Source data for Extended Data Fig. 5.
Source Data Extended Data Fig. 6
Source data for Extended Data Fig. 6.
Rights and permissions
About this article
Cite this article
Park, H., Park, H., Song, K. et al. In situ multiscale probing of the synthesis of a Ni-rich layered oxide cathode reveals reaction heterogeneity driven by competing kinetic pathways. Nat. Chem. 14, 614–622 (2022). https://doi.org/10.1038/s41557-022-00915-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-022-00915-2
This article is cited by
-
Re-evaluation of battery-grade lithium purity toward sustainable batteries
Nature Communications (2024)
-
High-Performance Thick Cathode Based on Polyhydroxyalkanoate Binder for Li Metal Batteries
Advanced Fiber Materials (2024)
-
Real-time observation of phase transition from layered to spinel phase under electron beam irradiation
Journal of Analytical Science and Technology (2023)
-
Eutectic salt-assisted planetary centrifugal deagglomeration for single-crystalline cathode synthesis
Nature Energy (2023)
-
Asynchronous domain dynamics and equilibration in layered oxide battery cathode
Nature Communications (2023)