Abstract
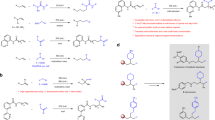
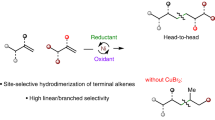
Primary alkyl halides have broad utility as fine chemicals in organic synthesis. The direct halogenation of alkenes is one of the most efficient approaches for the synthesis of these halides. Internal alkenes, in particular mixtures of isomers from refineries, constitute readily available and inexpensive feedstock and are the most attractive starting materials for this synthesis. However, the hydrohalogenation of alkenes generally affords branched alkyl halides; there are no catalytic methods to prepare linear alkyl halides directly from internal alkenes, let alone from a mixture of alkene isomers. Here we report the remote oxidative halogenation of alkenes under palladium catalysis via which both terminal and internal alkenes yield primary alkyl halides efficiently. Engineering pyridine-oxazoline ligands by introducing a hydroxyl group is essential for achieving excellent chemo- and regioselectivity. The catalytic system is also good for the mixture of alkene isomers generated from dehydrogenation of alkanes, providing a window to investigate the high-value utilization of inexpensive alkanes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the finding of this study are available in this article and the Supplementary Information. Crystallographic data for structures Pd(L6)Cl2, Pd(L6)2Cl2 and Pd(L9)Cl2 have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2026831, CCDC 2026793 and CCDC 2076726, respectively. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/
References
Weissermel, K. & Arpe, H.-J. Industrial Organic Chemistry 4th edn (Wiley-VCH, 2003).
Larock, R. C. Comprehensive Organic Transformations: A Guide to Functional Group Preparations 3rd edn, 1231−1467 (Wiley-VCH, 2018).
Gribble, G. W. in Progress in the Chemistry of Organic Natural Products Vol. 91 (Springer, 2010).
Hernandes, M. Z. et al. Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr. Drug Targets. 11, 303–314 (2010).
Tang, M. L. & Bao, Z. Halogenated materials as organic semiconductors. Chem. Mater. 23, 446–455 (2011).
Luh, T.-Y., Leung, M.-K. & Wong, K.-T. Transition metal-catalyzed activation of aliphatic C−X bonds in carbon−carbon bond formation. Chem. Rev. 100, 3187–3204 (2000).
Petrone, D. A., Ye, J. T. & Lautens, M. Modern transition-metal catalyzed carbon–halogen bond formation. Chem. Rev. 116, 8003–8104 (2016).
Varenikov, A., Shapiro, E. & Gandelman, M. Decarboxylative halogenation of organic compounds. Chem. Rev. 121, 412–484 (2021).
Agarwal, V. et al. Enzymatic halogenation and dehalogenation reactions: pervasive and mechanistically diverse. Chem. Rev. 117, 5619–5674 (2017).
El-Qisairi, A. K. & Henry, P. M. New palladium(II)-catalyzed asymmetric 1,2-dibromo synthesis. Org. Lett. 5, 439–441 (2003).
Denmark, S. E. & Carson, N. Reinvestigation of a catalytic, enantioselective alkene dibromination and chlorohydroxylation. Org. Lett. 17, 5728–5731 (2015).
Herron, A. N. & Yu, J.-Q. δ-C−H Mono- and dihalogenation of alcohols. J. Am. Chem. Soc. 142, 2766–2770 (2020).
Zhu, Y. & Yu, W. Visible-light-driven remote C−H chlorination of aliphatic sulamides with sodium hypochlorite. Asian J. Org. Chem. 9, 1650–1654 (2020).
Kharasch, M. S. & Kleiman, M. Synthesis of polyenes. III. A new synthesis of diethylstilbestrol. J. Am. Chem. Soc. 65, 11–15 (1943).
Kharasch, M. S. & Mayo, F. R. The peroxide effect in the addition of reagents to unsaturated compounds. I. The addition of hydrogen bromide to allyl bromide. J. Am. Chem. Soc. 55, 2468–2496 (1933).
Mayo, F. R. Free radical addition and transfer reactions of hydrogen chloride with unsaturated compounds. J. Am. Chem. Soc. 76, 5392–5396 (1954).
Wilger, D. J., Grandjean, J. M., Lammert, T. R. & Nicewicz, D. A. The direct anti-Markovnikov addition of mineral acids to styrenes. Nat. Chem. 6, 720–726 (2014).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Sommer, H., Juliá-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Bonfield, H. E., Valette, D., Lindsay, D. M. & Reid, M. Stereoselective remote functionalization via palladium-catalyzed redox-relay Heck methodologies. Chem. Eur. J. 27, 158–174 (2021).
Wu, L., Fleischer, I. & Beller, M. Ruthenium-catalyzed hydroformylation/reduction of olefins to alcohols: extending the scope to internal alkenes. J. Am. Chem. Soc. 135, 14306–14312 (2013).
Yuki, Y., Takahashi, K., Tanaka, Y. & Nozaki, K. Tandem isomerization/ hydroformylation/hydrogenation of internal alkenes to n-alcohols using Rh/Ru dual- or ternary-catalyst systems. J. Am. Chem. Soc. 135, 17393–17400 (2013).
Zhang, L., Peng, D., Leng, X. & Huang, Z. Iron-catalyzed, atom economical, chemo- and regioselective alkene hydroboration with pinacolborane. Angew. Chem. Int. Ed. 52, 3676–3680 (2013).
Sun, S.-Z., Borjesson, M., Martin-Montero, R. & Martin, R. Site-selective Ni-catalyzed reductive coupling of α-haloboranes with unactivated olefins. J. Am. Chem. Soc. 140, 12765–12769 (2018).
Juliá-Hernández, F., Moragas, T., Cornella, J. & Martin, R. Remote carboxylation of halogenated aliphatic hydrocarbons with carbon dioxide. Nature 545, 84–88 (2017).
Bhunia, A., Bergander, K. & Studer, A. Cooperative palladium/Lewis acid-catalyzed transfer hydrocyanation of alkenes and alkynes using 1-methylcyclohexa-2,5-diene-1-carbonitrile. J. Am. Chem. Soc. 140, 16353–16359 (2018).
Werner, E. W., Mei, T.-S., Burckle, A. J. & Sigman, M. S. Enantioselective Heck arylations of acyclic alkenyl alcohols using a redox-relay strategy. Science 338, 1455–1458 (2012).
Mei, T.-S., Patel, H. H. & Sigman, M. S. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014).
Ho, G.-M., Jiudkele, L., Bruffaerts, J. & Marek, I. Metal-catalyzed remote functionalization of ω-ene unsaturated ethers as a new entry to functionalized vinyl species. Angew. Chem. Int. Ed. 57, 8012–8016 (2018).
Sehnal, P., Taylor, R. J. K. & Fairlamb, I. J. S. Emergence of palladium(IV) chemistry in synthesis and catalysis. Chem. Rev. 110, 824–889 (2010).
Hickman, A. J. & Sanford, M. S. High-valent organometallic copper and palladium in catalysis. Nature 484, 177–185 (2012).
Yin, G., Mu, X. & Liu, G. Palladium-catalyzed oxidative difunctonalization of alkenes: bond forming at a high-valent palladium center. Acc. Chem. Res. 49, 2413–2423 (2016).
Qi, X. et al. Enantioselective Pd(II)-catalyzed intramolecular oxidative 6-endo-amino-acetoxylation of unactivated alkenes. J. Am. Chem. Soc. 140, 7415–7419 (2018).
Tian, B., Chen, P., Leng, X. & Liu, G. Palladium-catalyzed enantioselective diactoxylation of terminal alkenes. Nat. Catal. 4, 172–179 (2021).
Garces, K. et al. Iridium-catalyzed hydrogen production from hydrosilanes and water. ChemCatChem 6, 1691–1697 (2014).
Pendleton, I. M., Pérez-Temprano, M. H., Sanford, M. S. & Zimmerman, P. M. Experimental and computational assessment of reactivity and mechanism in C(sp3)−N bond-forming reductive elimination from palladium(iv). J. Am. Chem. Soc. 138, 6049–6060 (2016).
Cai, Y. F. et al. Highly enantioselective α-chlorination of cyclic β-ketoesters catalyzed by N,N-dioxide using NCS as the chlorine source. Chem. Commun. 46, 1250–1252 (2010).
Saikia, I., Borah, A. J. & Phukan, P. Use of bromine and bromo-organic compounds in organic synthesis. Chem. Rev. 116, 6837–7042 (2016).
He, Y., Cai, Y. & Zhu, S. Mild and regioselective benzylic C−H functionalization: Ni-catalyzed reductive arylation of remote and proximal olefins. J. Am. Chem. Soc. 139, 1061–1064 (2017).
Wang, W. et al. Migratory arylboration of unactivated alkenes enabled by nickel catalysis. Angew. Chem. Int. Ed. 58, 4612–4616 (2019).
Liu, F. & Goldman, A. S. Efficient thermochemical alkane dehydrogenation and isomerization catalyzed by an iridium pincer complex. Chem. Commun. 655−656 (1999).
Acknowledgements
Financial Support was provided by the National Key R&D Program of China (No. 2021YFA1500100), the National Natural Science Foundation of China (numbers 91956202, 21971255, 21790330 and 21821002), the Science and Technology Commission of Shanghai Municipality (numbers 20JC1417000, 21520780100 and 19590750400), the International Partnership Program (number 121731KYSB20190016), and the Key Research Program of Frontier Science (number QYZDJSSW-SLH055) of the Chinese Academy of Sciences. P.C. also thanks the Youth Innovation Promotion Association of the Chinese Academy of Sciences (number 2018292).
Author information
Authors and Affiliations
Contributions
X.L., J.J. and G.L. conceived the work and designed the experiments. X.L. and J.J. performed the laboratory experiments. X.L., P.C. and G.L. analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
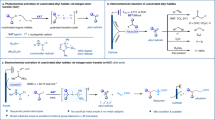
Extended Data Fig. 1 The function of NEt3.
a. The side reactions when L9 was employed in the reaction. b. The possible mechanism for the side product formation. The oxygenation and amination product were formed via reductive elimination from PdIV. c. NEt3 acted as a liable ligand to accelerate the dissociation chloride from Pd IV intermediate, which was helpful for the formation of C–Cl bond.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, Figs. 1–6, methods, mechanism experiments, data and references.
Supplementary Data 1
Crystallographic data for compound (L6)2PdCl2. CCDC reference 2026793.
Supplementary Data 2
Crystallographic data for compound (L6)PdCl2. CCDC reference 2026831.
Supplementary Data 3
Crystallographic data for compound (L9)PdCl2. CCDC reference 2076726.
Rights and permissions
About this article
Cite this article
Li, X., Jin, J., Chen, P. et al. Catalytic remote hydrohalogenation of internal alkenes. Nat. Chem. 14, 425–432 (2022). https://doi.org/10.1038/s41557-021-00869-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00869-x
This article is cited by
-
Tuning light-driven oxidation of styrene inside water-soluble nanocages
Nature Communications (2024)
-
Anti-Markovnikov hydrochlorination and hydronitrooxylation of α-olefins via visible-light photocatalysis
Nature Catalysis (2023)
-
Multi-site programmable functionalization of alkenes via controllable alkene isomerization
Nature Chemistry (2023)