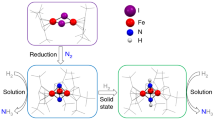
Abstract
Iron nitrides are key intermediates in biological nitrogen fixation and the industrial Haber–Bosch process, used to form ammonia from dinitrogen. However, the proposed successive conversion of nitride to ammonia remains elusive. In this regard, the search for well-described multi-iron nitrido model complexes and investigations on controlling their reactivity towards ammonia formation have long been of great challenge and importance. Here we report a well-defined thiolate-bridged FeIVFeIV μ-nitrido complex featuring an uncommon bent Fe–N–Fe moiety. Remarkably, this complex shows excellent reactivity toward hydrogenation with H2 at ambient conditions, forming ammonia in high yield. Combined experimental and computational studies demonstrate that a thiolate-bridged FeIIIFeIII μ-amido complex is a key intermediate, which is generated through an unusual two-electron oxidation of H2. Moreover, ammonia production was also realized by treating this diiron μ-nitride with electrons and water as a proton source.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data resulting from the experimental and computational studies of this work are included within this Article and the Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1400911 (2[PF6]·THF), 1435241 (3[BPh4] at 100 K), 2108184 (3[BPh4] at 298 K), 1402944 (4[BPh4]·0.5THF), 1402945 (5[BPh4]) and 2089536 (6). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Liu, H. Ammonia synthesis catalyst 100 years: practice, enlightenment and challenge. Chin. J. Catal. 35, 1619–1640 (2014).
Hohenberger, J., Ray, K. & Meyer, K. The biology and chemistry of high-valent iron–oxo and iron–nitrido complexes. Nat. Commun. 3, 720–732 (2012).
Buscagan, T. M. & Rees, D. C. Rethinking the nitrogenase mechanism: activating the active site. Joule 3, 2662–2678 (2019).
Ertl, G. Reactions at surfaces: from atoms to complexity (Nobel lecture). Angew. Chem. Int. Ed. 47, 3524–3535 (2008).
Schlögl, R. in Handbook of Heterogeneous Catalysis (eds Ertl, G. et al.) 2501−2575 (Wiley-VCH, 2008).
Seefeldt, L. C. et al. Reduction of substrates by nitrogenases. Chem. Rev. 120, 5082–5106 (2020).
Spatzal, T., Perez, K. A., Einsle, O., Howard, J. B. & Rees, D. C. Ligand binding to the FeMo-cofactor: structures of CO-bound and reactivated nitrogenase. Science 345, 1620–1623 (2014).
Sippel, D. et al. A bound reaction intermediate sheds light on the mechanism of nitrogenase. Science 359, 1484–1489 (2018).
Kang, W., Lee, C. C., Jasniewski, A. J., Ribbe, M. W. & Hu, Y. Structural evidence for a dynamic metallocofactor during N2 reduction by Mo-nitrogenase. Science 368, 1381–1385 (2020).
Jüstel, T. et al. μ-Nitridodiiron complexes with asymmetric [FeIV≡N−FeIII]4+ and symmetric [FeIV=N=FeIV]5+ structural elements. Angew. Chem. Int. Ed. 34, 669–672 (1995).
Brown, S. D. & Peters, J. C. Ground-state singlet L3Fe-(μ-N)-FeL3 and L3Fe(NR) complexes featuring pseudotetrahedral Fe(II) centers. J. Am. Chem. Soc. 127, 1913–1923 (2005).
Kudrik, E. V. et al. An N-bridged high-valent diiron-oxo species on a porphyrin platform that can oxidize methane. Nat. Chem. 4, 1024–1029 (2012).
Reiners, M. et al. Reactivity studies on [Cp′Fe(μ-I)]2: nitrido-, sulfide- and diselenide iron complexes derived from pseudohalide activation. Chem. Sci. 8, 4108–4122 (2017).
Zhang, S. et al. N−H bond formation at a diiron bridging nitride. Angew. Chem. Int. Ed. 59, 15215–15219 (2020).
Bennett, M. V., Stoian, S., Bominaar, E. L., Münck, E. & Holm, R. H. Initial members of the family of molecular mid-valent high-nuclearity iron nitrides: [Fe4N2X10]4− and [Fe10N8X12]5− (X = Cl−, Br−). J. Am. Chem. Soc. 127, 12378–12386 (2005).
Powers, T. M., Fout, A. R., Zheng, S.-L. & Betley, T. A. Oxidative group transfer to a triiron complex to form a nucleophilic μ3-nitride, [Fe3(μ3-N)]−. J. Am. Chem. Soc. 133, 3336–3338 (2011).
Rodriguez, M. M., Bill, E., Brennessel, W. W. & Holland, P. L. N2 reduction and hydrogenation to ammonia by a molecular iron–potassium complex. Science 334, 780–783 (2011).
Ermert, D. M., Gordon, J. B., Abboud, K. A. & Murray, L. J. Nitride-bridged triiron complex and its relevance to dinitrogen activation. Inorg. Chem. 54, 9282–9289 (2015).
Drance, M. J. et al. Controlled expansion of a strong-field iron nitride cluster: multi-site ligand substitution as a strategy for activating interstitial nitride nucleophilicity. Angew. Chem. Int. Ed. 57, 13057–13061 (2018).
Reiners, M. et al. NH3 formation from N2 and H2 mediated by molecular tri-iron complexes. Nat. Chem. 12, 740–746 (2020).
MacLeod, K. C., McWilliams, S. F., Mercado, B. Q. & Holland, P. L. Stepwise N–H bond formation from N2-derived iron nitride, imide and amide intermediates to ammonia. Chem. Sci. 7, 5736–5746 (2016).
Brown, S. D., Mehn, M. P. & Peters, J. C. Heterolytic H2 activation mediated by low-coordinate L3Fe-(μ-N)-FeL3 complexes to generate Fe(μ-NH)(μ-H)Fe species. J. Am. Chem. Soc. 127, 13146–13147 (2005).
Tanifuji, K. & Ohki, Y. Metal–sulfur compounds in N2 reduction and nitrogenase-related chemistry. Chem. Rev. 120, 5194–5251 (2020).
Chen, Y. et al. Nitrogenase model complexes [Cp*Fe(μ-SR1)2(μ-η2-R2N=NH)FeCp*] (R1 = Me, Et; R2 = Me, Ph; Cp* = η5-C5Me5): synthesis, structure and catalytic N−N bond cleavage of hydrazines on diiron centers. J. Am. Chem. Soc. 130, 15250–15251 (2008).
Chen, Y. et al. Unusual thiolate-bridged diiron clusters bearing the cis-HN=NH ligand and their reactivities with terminal alkynes. J. Am. Chem. Soc. 133, 1147–1149 (2011).
Li, Y. et al. Ammonia formation by a thiolate-bridged diiron amide complex as a nitrogenase mimic. Nat. Chem. 5, 320–326 (2013).
Pappas, I. & Chirik, P. J. Catalytic proton coupled electron transfer from metal hydrides to titanocene amides, hydrazides and imides: determination of thermodynamic parameters relevant to nitrogen fixation. J. Am. Chem. Soc. 138, 13379–13389 (2016).
Spatzal, T. et al. Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science 334, 940 (2011).
Lancaster, K. M. et al. X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron-molybdenum cofactor. Science 334, 974–977 (2011).
Jüstel, T. et al. The molecular and electronic structure of symmetrically and asymmetrically coordinated, non-heme iron complexes containing [FeIII(μ-N)FeIV]4+ (S = 3/2) and [FeIV(μ-N)FeIV]5+ (S = 0) cores. Chem. Eur. J. 5, 793–810 (1999).
Vitillo, J. G., Bhan, A., Cramer, C. J., Lu, C. C. & Gagliardi, L. Quantum chemical characterization of structural single Fe(II) sites in MIL-type metal−organic frameworks for the oxidation of methane to methanol and ethane to ethanol. ACS Catal. 9, 2870–2879 (2019).
Gaggioli, C. A., Stoneburner, S. J., Cramer, C. J. & Gagliardi, L. Beyond density functional theory: the multiconfigurational approach to model heterogeneous catalysis. ACS Catal. 9, 8481–8502 (2019).
Ghosh, M. et al. A μ-phosphido diiron dumbbell in multiple oxidation states. Angew. Chem. Int. Ed. 58, 14349–14356 (2019).
Saouma, C. T., Müller, P. & Peters, J. C. Characterization of structurally unusual diiron NxHy complexes. J. Am. Chem. Soc. 131, 10358–10359 (2009).
Askevold, B. et al. Ammonia formation by metal–ligand cooperative hydrogenolysis of a nitrido ligand. Nat. Chem. 3, 532–537 (2011).
Schendzielorz, F. S., Finger, M., Volkmann, C., Würtele, C. & Schneider, S. A terminal osmium(IV) nitride: ammonia formation and ambiphilic reactivity. Angew. Chem. Int. Ed. 55, 11417–11420 (2016).
Falcone, M., Chatelain, L., Scopelliti, R., Živković, I. & Mazzanti, M. Nitrogen reduction and functionalization by a multimetallic uranium nitride complex. Nature 547, 332–335 (2017).
Kim, S., Zhong, H., Park, Y., Loose, F. & Chirik, P. J. Catalytic hydrogenation of a manganese(V) nitride to ammonia. J. Am. Chem. Soc. 142, 9518–9524 (2020).
Hidai, M. & Mizobe, Y. Recent advances in the chemistry of dinitrogen complexes. Chem. Rev. 95, 1115–1133 (1995).
Yandulov, D. V. & Schrock, R. R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 301, 76–78 (2003).
Scepaniak, J. J. et al. Synthesis, structure, and reactivity of an iron(V) nitride. Science 331, 1049–1052 (2011).
King, D. M. et al. Synthesis and structure of a terminal uranium nitride complex. Science 337, 717–720 (2012).
Ashida, Y., Arashiba, K., Nakajima, K. & Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 568, 536–540 (2019).
Anderson, J. S., Rittle, J. & Peters, J. C. Catalytic conversion of nitrogen to ammonia by an iron model complex. Nature 501, 84–88 (2013).
Chen, Y., Zhou, Y. & Qu, J. Synthesis and reactions of novel triply thiolate-bridged diiron complexes [Cp*Fe(μ2-SR)3FeCp*] (Cp* = η5-C5Me5; R = Et, Ph). Organometallics 27, 666–671 (2008).
Büchner, R., Field, J. S. & Haines, R. J. Electron-transfer salts derived from i,N′-dicyano-p-benzoquinone diimines and the novel dinuclear organometallic donor [Fe2(η-C5Me5)2(μ-SEt)2(CO)2]. J. Chem. Soc. Dalton Trans. 1996, 3533–3538 (1996).
Shima, T. et al. Dinitrogen cleavage and hydrogenation by a trinuclear titanium polyhydride complex. Science 340, 1549–1552 (2013).
Chatelain, L. et al. Terminal uranium(V)-nitride hydrogenations involving direct addition or frustrated Lewis pair mechanisms. Nat. Commun. 11, 337 (2020).
Bullock, R. M. & Helm, M. L. Molecular electrocatalysts for oxidation of hydrogen using earth-abundant metals: shoving protons around with proton relays. Acc. Chem. Res. 48, 2017–2026 (2015).
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 110, 6961–7001 (2010).
Weinberg, D. R. et al. Proton-coupled electron transfer. Chem. Rev. 112, 4016–4093 (2012).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants nos. 21690064, 22001031 and 21231003), Key Laboratory of Bio-based Chemicals of Liaoning Province of China and the ‘111’ project of the Ministry of Education of China. J.Q. would like to especially thank M. Hidai from the University of Tokyo for his continuous guidance and valuable support. We would also like to express our gratitude to E. Bill and B. Mienert of the Max-Planck Institute for Chemical Energy Conversion (MPI-CEC) for help with the acquisition of the Mössbauer data, as well as for fruitful discussions.
Author information
Authors and Affiliations
Contributions
J.Q. and B.W. supervised the project. J.Q. and J.Z. conceived and designed the experiments. Y. Zhang, J.Z., D.Y. and T.M. performed the experiments. J.W. and S.Y. performed the Mössbauer measurements. S.Y. and H.C. carried out quantum chemical calculations. J.Q., S.Y., B.W., D.Y. and Y. Zhang co-wrote the paper. S.Y., Y. Zhou, J.Z., D.Y. and Y. Zhang analysed the data. All authors discussed the results in detail and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–46, Tables 1–19 and Discussion.
Supplementary Data 1
Crystallographic data for compound 2[PF6]·THF. CCDC reference 1400911.
Supplementary Data 2
Crystallographic data for compound 3[BPh4] at 100 K. CCDC reference 1435241.
Supplementary Data 3
Crystallographic data for compound 3[BPh4] at 298 K. CCDC reference 2108184.
Supplementary Data 4
Crystallographic data for compound 4[BPh4]·0.5THF. CCDC reference 1402944.
Supplementary Data 5
Crystallographic data for compound 5[BPh4]. CCDC reference 1402945.
Supplementary Data 6
Crystallographic data for compound 6. CCDC reference 2089536.
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhao, J., Yang, D. et al. A thiolate-bridged FeIVFeIV μ-nitrido complex and its hydrogenation reactivity toward ammonia formation. Nat. Chem. 14, 46–52 (2022). https://doi.org/10.1038/s41557-021-00852-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00852-6
This article is cited by
-
Potassium Promoted Ferrocene/Graphene for Ammonia Synthesis
Chemical Research in Chinese Universities (2024)
-
Order–order assembly transition-driven polyamines detection based on iron−sulfur complexes
Communications Chemistry (2023)
-
Ammonia formation revisited
Nature Chemistry (2022)