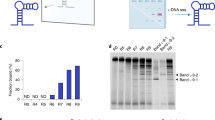
Abstract
DNA-encoded library technologies enable the screening of synthetic molecules but have thus far not tapped into the power of Darwinian selection with iterative cycles of selection, amplification and diversification. Here we report a simple strategy to rapidly assemble libraries of conformationally constrained peptides that are paired in a combinatorial fashion (suprabodies). We demonstrate that the pairing can be shuffled after each amplification cycle in a process similar to DNA shuffling or mating to regenerate diversity. Using simulations, we show the benefits of this recombination in yielding a more accurate correlation of selection fitness with affinity after multiple rounds of selection, particularly if the starting library is heterogeneous in the concentration of its members. The method was validated with selections against streptavidin and applied to the discovery of PD-L1 binders. We further demonstrate that the binding of self-assembled suprabodies can be recapitulated by smaller (∼7 kDa) synthetic products that maintain the conformational constraint of the peptides.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within this paper and its Supplementary Information. Raw data has been deposited on Zenodo (https://doi.org/10.5281/zenodo.5011513). Source data are provided with this paper.
References
Neri, D. & Lerner, R. A. DNA-encoded chemical libraries: a selection system based on endowing organic compounds with amplifiable information. Annu. Rev. Biochem. 87, 479–502 (2018).
Goodnow, R. A., Dumelin, C. E. & Keefe, A. D. DNA-encoded chemistry: enabling the deeper sampling of chemical space. Nat. Rev. Drug Discov. 16, 131–147 (2017).
Brenner, S. & Lerner, R. A. Encoded combinatorial chemistry. Proc. Natl Acad. Sci. USA 89, 5381–5383 (1992).
Gartner, Z. J. et al. DNA-templated organic synthesis and selection of a library of macrocycles. Science 305, 1601–1605 (2004).
Georghiou, G., Kleiner, R. E., Pulkoski-Gross, M., Liu, D. R. & Seeliger, M. A. Highly specific, bisubstrate-competitive Src inhibitors from DNA-templated macrocycles. Nat. Chem. Biol. 8, 366–374 (2012).
Harris, J. L. & Winssinger, N. PNA encoding (PNA = peptide nucleic acid): from solution-based libraries to organized microarrays. Chem. Eur. J. 11, 6792–6801 (2005).
Zambaldo, C., Barluenga, S. & Winssinger, N. PNA-encoded chemical libraries. Curr. Opin. Chem. Biol. 26, 8–15 (2015).
Wrenn, S. J., Weisinger, R. M., Halpin, D. R. & Harbury, P. B. Synthetic ligands discovered by in vitro selection. J. Am. Chem. Soc. 129, 13137–13143 (2007).
Weisinger, R. M., Wrenn, S. J. & Harbury, P. B. Highly parallel translation of DNA sequences into small molecules. PLoS ONE 7, e28056 (2012).
Melkko, S., Scheuermann, J., Dumelin, C. E. & Neri, D. Encoded self-assembling chemical libraries. Nat. Biotechnol. 22, 568–574 (2004).
Mannocci, L. et al. High-throughput sequencing allows the identification of binding molecules isolated from DNA-encoded chemical libraries. Proc. Natl Acad. Sci. USA 105, 17670–17675 (2008).
Clark, M. A. et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 5, 647–654 (2009).
Daguer, J. P., Ciobanu, M., Alvarez, S., Barluenga, S. & Winssinger, N. DNA-templated combinatorial assembly of small molecule fragments amenable to selection/amplification cycles. Chem. Sci. 2, 625–632 (2011).
Ciobanu, M. et al. Selection of a synthetic glycan oligomer from a library of DNA-templated fragments against DC-SIGN and inhibition of HIV gp120 binding to dendritic cells. Chem. Commun. 47, 9321–9323 (2011).
Hook, K. D., Chambers, J. T. & Hili, R. A platform for high-throughput screening of DNA-encoded catalyst libraries in organic solvents. Chem. Sci. 8, 7072–7076 (2017).
Usanov, D. L., Chan, A. I., Maianti, J. P. & Liu, D. R. Second-generation DNA-templated macrocycle libraries for the discovery of bioactive small molecules. Nat. Chem. 10, 704–714 (2018).
Brudno, Y., Birnbaum, M. E., Kleiner, R. E. & Liu, D. R. An in vitro translation, selection and amplification system for peptide nucleic acids. Nat. Chem. Biol. 6, 148–155 (2010).
Niu, J., Hili, R. & Liu, D. R. Enzyme-free translation of DNA into sequence-defined synthetic polymers structurally unrelated to nucleic acids. Nat. Chem. 5, 282–292 (2013).
Krusemark, C. J., Tilmans, N. P., Brown, P. O. & Harbury, P. B. Directed chemical evolution with an outsized genetic code. PLoS ONE 11, e0154765 (2016).
Kong, D. H., Yeung, W. & Hili, R. In vitro selection of diversely functionalized aptamers. J. Am. Chem. Soc. 139, 13977–13980 (2017).
Chen, Z., Lichtor, P. A., Berliner, A. P., Chen, J. C. & Liu, D. R. Evolution of sequence-defined highly functionalized nucleic acid polymers. Nat. Chem. 10, 420–427 (2018).
Stemmer, W. P. C. Rapid evolution of a protein in-vitro by DNA shuffling. Nature 370, 389–391 (1994).
Crameri, A., Raillard, S. A., Bermudez, E. & Stemmer, W. P. C. DNA shuffling of a family of genes from diverse species accelerates directed evolution. Nature 391, 288–291 (1998).
Smith, G. P. Applied evolution—the progeny of sexual PCR. Nature 370, 324–325 (1994).
Melkko, S., Zhang, Y., Dumelin, C. E., Scheuermann, J. & Neri, D. Isolation of high-affinity trypsin inhibitors from a DNA-encoded chemical library. Angew. Chem. Int. Ed. 46, 4671–4674 (2007).
Wichert, M. et al. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat. Chem. 7, 241–249 (2015).
Li, G. et al. Design, preparation, and selection of DNA-encoded dynamic libraries. Chem. Sci. 6, 7097–7104 (2015).
Zimmermann, G. et al. A specific and covalent JNK-1 ligand selected from an encoded self-assembling chemical library. Chem. Eur. J. 23, 8152–8155 (2017).
Zhou, Y. et al. DNA-encoded dynamic chemical library and its applications in ligand discovery. J. Am. Chem. Soc. 140, 15859–15867 (2018).
Reddavide, F. V. et al. Second generation DNA-encoded dynamic combinatorial chemical libraries. Chem. Commun. 55, 3753–3756 (2019).
Figuerola-Conchas, A. et al. Small-molecule modulators of the ATPase VCP/p97 affect specific p97 cellular functions. ACS Chem. Biol. 15, 243–253 (2020).
Deng, Y. Q. et al. Selection of DNA-encoded dynamic chemical libraries for direct inhibitor discovery. Angew. Chem. Int. Ed. 59, 14965–14972 (2020).
Driggers, E. M., Hale, S. P., Lee, J. & Terrett, N. K. The exploration of macrocycles for drug discovery—an underexploited structural class. Nat. Rev. Drug Discov. 7, 608–624 (2008).
Zorzi, A., Deyle, K. & Heinis, C. Cyclic peptide therapeutics: past, present and future. Curr. Opin. Chem. Biol. 38, 24–29 (2017).
Li, Y. Z. et al. Versatile protein recognition by the encoded display of multiple chemical elements on a constant macrocyclic scaffold. Nat. Chem. 10, 441–448 (2018).
Tan, X., Bruchez, M. P. & Armitage, B. A. Closing the loop: constraining TAT peptide by γPNA hairpin for enhanced cellular delivery of biomolecules. Bioconjug. Chem. 29, 2892–2898 (2018).
Machida, T., Dutt, S. & Winssinger, N. Allosterically regulated phosphatase activity from peptide–PNA conjugates folded through hybridization. Angew. Chem. Int. Ed. 55, 8595–8598 (2016).
Barluenga, S. & Winssinger, N. PNA as a biosupramolecular tag for programmable assemblies and reactions. Accounts Chem Res 48, 1319–1331 (2015).
Li, X. Y. & Liu, D. R. DNA-templated organic synthesis: nature’s strategy for controlling chemical reactivity applied to synthetic molecules. Angew. Chem. Int. Ed. 43, 4848–4870 (2004).
Berteotti, A. et al. Predicting the reactivity of nitrile-carrying compounds with cysteine: a combined computational and experimental study. ACS Med. Chem. Lett. 5, 501–505 (2014).
Ramil, C. P., An, P., Yu, Z. & Lin, Q. Sequence-specific 2-cyanobenzothiazole ligation. J. Am. Chem. Soc. 138, 5499–5502 (2016).
Nitsche, C. et al. Biocompatible macrocyclization between cysteine and 2-cyanopyridine generates stable peptide inhibitors. Org. Lett. 21, 4709–4712 (2019).
Todorovic, M. et al. Fluorescent isoindole crosslink (FlICk) chemistry: a rapid, user-friendly stapling reaction. Angew. Chem. Int. Ed. 58, 14120–14124 (2019).
Zhang, Y., Zhang, Q., Wong, C. T. T. & Li, X. C. Chemoselective peptide cyclization and bicyclization directly on unprotected peptides. J. Am. Chem. Soc. 141, 12274–12279 (2019).
Schmidt, T. G. M., Koepke, J., Frank, R. & Skerra, A. Molecular interaction between the Strep-tag affinity peptide and its cognate target, streptavidin. J. Mol. Biol. 255, 753–766 (1996).
Busby, M., Stadler, L. K. J., Ferrigno, P. K. & Davis, J. J. Optimisation of a multivalent Strep tag for protein detection. Biophys. Chem. 152, 170–177 (2010).
Freeman, G. J. et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 192, 1027–1034 (2000).
Zak, K. M. et al. Structure of the complex of human programmed death 1, PD-1, and its ligand PD-L1. Structure 23, 2341–2348 (2015).
Caldwell, C. et al. Identification and validation of a PD-L1 binding peptide for determination of PDL1 expression in tumors. Sci. Rep. 7, 13682 (2017).
Touti, F., Gates, Z. P., Bandyopdhyay, A., Lautrette, G. & Pentelute, B. L. In-solution enrichment identifies peptide inhibitors of protein–protein interactions. Nat. Chem. Biol. 15, 410–418 (2019).
Shaabani, S. et al. A patent review on PD-1/PD-L1 antagonists: small molecules, peptides, and macrocycles (2015–2018). Expert Opin. Ther. Pat. 28, 665–678 (2018).
Satz, A. L. DNA encoded library selections and insights provided by computational simulations. ACS Chem. Biol. 10, 2237–2245 (2015).
Satz, A. L., Hochstrasser, R. & Petersen, A. C. Analysis of current DNA encoded library screening data indicates higher false negative rates for numerically larger libraries. ACS Comb. Sci. 19, 234–238 (2017).
Smith, G. P. Filamentous fusion phage—novel expression vectors that display cloned antigens on the virion surface. Science 228, 1315–1317 (1985).
Ellington, A. D. & Szostak, J. W. Invitro selection of RNA Molecules that bind specific ligands. Nature 346, 818–822 (1990).
Tuerk, C. & Gold, L. Systematic evolution of ligands by exponential enrichment—RNA ligands to bacteriophage-T4 DNA-polymerase. Science 249, 505–510 (1990).
Roberts, R. W. & Szostak, J. W. RNA–peptide fusions for the in vitro selection of peptides and proteins. Proc. Natl Acad. Sci. USA 94, 12297–12302 (1997).
Kale, S. S. et al. Cyclization of peptides with two chemical bridges affords large scaffold diversities. Nat. Chem. 10, 715–723 (2018).
Heinis, C., Rutherford, T., Freund, S. & Winter, G. Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat. Chem. Biol. 5, 502–507 (2009).
Navaratna, T. et al. Directed evolution using stabilized bacterial peptide display. J. Am. Chem. Soc. 142, 1882–1894 (2020).
Huang, Y. C., Wiedmann, M. M. & Suga, H. RNA display methods for the discovery of bioactive macrocycles. Chem. Rev. 119, 10360–10391 (2019).
Lee, J. et al. Expanding the limits of the second genetic code with ribozymes. Nat. Commun. 10, 5097 (2019).
Vinogradov, A. A. et al. Minimal lactazole scaffold for in vitro thiopeptide bioengineering. Nat. Commun. 11, 2272 (2020).
Heinis, C. & Winter, G. Encoded libraries of chemically modified peptides. Curr. Opin. Chem. Biol. 26, 89–98 (2015).
Josephson, K., Ricardo, A. & Szostak, J. W. mRNA display: from basic principles to macrocycle drug discovery. Drug Discov. Today 19, 388–399 (2014).
Obexer, R., Walport, L. J. & Suga, H. Exploring sequence space: harnessing chemical and biological diversity towards new peptide leads. Curr. Opin. Chem. Biol. 38, 52–61 (2017).
Goto, Y., Katoh, T. & Suga, H. Flexizymes for genetic code reprogramming. Nat. Protoc. 6, 779–790 (2011).
Acknowledgements
This work was supported by SNSF grants (169141 and 188406) and the NCCR chemical biology (185898). We thank V. Dutoit (Laboratory of Tumor Immunology, Faculty of Medicine, University of Geneva) for providing the U251 cell line.
Author information
Authors and Affiliations
Contributions
N.W. conceived the technology. B.R.V., L.F.-S., J-.P.D., S.B. and N.W. designed the experiments, analysed data and wrote the manuscript. B.R.V established and performed the selections. L.F.-S. established the chemical ligations. J.-P.D. designed the sequences of the library. B.R.V, L.F.-S. and M.D. synthesized and characterized the hits after selection.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Ryan Hili, Thomas Kodadek, Alexander Satz and Yixin Zhang for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary information.
Supplementary Table 1
Mathematical simulations.
Source data
Source Data Fig. 2
Unprocessed gels for Fig. 2c.
Source Data Fig. 3
Unprocessed gels for Fig. 3a,b.
Source Data Fig. 4
Data for graphs in Fig. 4b–d.
Source Data Fig. 5
Data for graphs in Fig. 5c,e.
Source Data Fig. 6
Data for graphs in Fig. 6d–e.
Source Data Fig. 7
Data for graphs in Fig. 7a–c,e,f.
Rights and permissions
About this article
Cite this article
Vummidi, B.R., Farrera-Soler, L., Daguer, JP. et al. A mating mechanism to generate diversity for the Darwinian selection of DNA-encoded synthetic molecules. Nat. Chem. 14, 141–152 (2022). https://doi.org/10.1038/s41557-021-00829-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00829-5
This article is cited by
-
Protein-templated ligand discovery via the selection of DNA-encoded dynamic libraries
Nature Chemistry (2024)
-
Trio-pharmacophore DNA-encoded chemical library for simultaneous selection of fragments and linkers
Nature Communications (2023)