Abstract
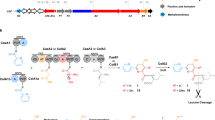
Aromatic amines in nature are typically installed with Glu or Gln as the nitrogen donor. Here we report a pathway that features glycyl-tRNA instead. During the biosynthesis of pyrroloiminoquinone-type natural products such as ammosamides, peptide-aminoacyl tRNA ligases append amino acids to the C-terminus of a ribosomally synthesized peptide. First, \({\mathrm{Amm}}{{{\mathrm{B}}}}_{{{\mathrm{C}}}}^{{{{\mathrm{Trp}}}}}\) adds Trp in a Trp-tRNA-dependent reaction and the flavoprotein AmmC1 then carries out three hydroxylations of the indole ring of Trp. After oxidation to the corresponding ortho-hydroxy para-quinone, \({\mathrm{Amm}}{{{\mathrm{B}}}}_{{{\mathrm{D}}}}^{{{{\mathrm{Gly}}}}}\) attaches Gly to the indole ring in a Gly-tRNA dependent fashion. Subsequent decarboxylation and hydrolysis results in an amino-substituted indole. Similar transformations are catalysed by orthologous enzymes from Bacillus halodurans. This pathway features three previously unknown biochemical processes using a ribosomally synthesized peptide as scaffold for non-ribosomal peptide extension and chemical modification to generate an amino acid-derived natural product.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lin, S. et al. Another look at pyrroloiminoquinone alkaloids-perspectives on their therapeutic potential from known structures and semisynthetic analogues. Marine Drugs 15, 98 (2017).
Peters, S. & Spiteller, P. Sanguinones A and B, blue pyrroloquinoline alkaloids from the fruiting bodies of the mushroom Mycena sanguinolenta. J. Nat. Prod. 70, 1274–1277 (2007).
Legentil, L., Benel, L., Bertrand, V., Lesur, B. & Delfourne, E. Synthesis and antitumor characterization of pyrazolic analogues of the marine pyrroloquinoline alkaloids: wakayin and tsitsikammamines. J. Med. Chem. 49, 2979–2988 (2006).
Hu, J. F., Fan, H., Xiong, J. & Wu, S. B. Discorhabdins and pyrroloiminoquinone-related alkaloids. Chem. Rev. 111, 5465–5491 (2011).
Chen, Q. B., Xin, X. L., Yang, Y., Lee, S. S. & Aisa, H. A. Highly conjugated norditerpenoid and pyrroloquinoline alkaloids with potent PTP1B iinhibitory activity from Nigella glandulifera. J. Nat. Prod. 77, 807–812 (2014).
Hughes, C. C., MacMillan, J. B., Gaudencio, S. P., Jensen, P. R. & Fenical, W. The ammosamides: structures of cell cycle modulators from a marine-derived Streptomyces species. Angew. Chem. Int. Ed. 48, 725–727 (2009).
Jordan, P. A. & Moore, B. S. Biosynthetic pathway connects cryptic ribosomally synthesized posttranslationally modified peptide genes with pyrroloquinoline alkaloids. Cell Chem. Biol. 23, 1504–1514 (2016).
Reimer, D. & Hughes, C. C. Thiol-based probe for electrophilic natural products reveals that most of the ammosamides are artifacts. J. Nat. Prod. 80, 126–133 (2017).
Reddy, P. V., Banerjee, B. & Cushman, M. Efficient total synthesis of ammosamide B. Org. Lett. 12, 3112–3114 (2010).
Hughes, C. C., MacMillan, J. B., Gaudencio, S. P., Fenical, W. & La Clair, J. J. Ammosamides A and B target myosin. Angew. Chem. Int. Ed. Engl. 48, 728–732 (2009).
Luo, J. et al. Discovery of ammosesters by mining the Streptomyces uncialis DCA2648 genome revealing new insight into ammosamide biosynthesis. J. Ind. Microbiol. Biotechnol. 48, kuab027 (2021).
Miyanaga, A. et al. Discovery and assembly-line biosynthesis of the lymphostin pyrroloquinoline alkaloid family of mTOR inhibitors in Salinispora bacteria. J. Am. Chem. Soc. 133, 13311–13313 (2011).
Colosimo, D. A. & MacMillan, J. B. Ammosamides unveil novel biosynthetic machinery. Cell Chem. Biol. 23, 1444–1446 (2016).
Ting, C. P. et al. Use of a scaffold peptide in the biosynthesis of amino acid-derived natural products. Science 365, 280–284 (2019).
Ortega, M. A. et al. Structure and mechanism of the tRNA-dependent lantibiotic dehydratase NisB. Nature 517, 509–512 (2015).
Garg, N., Salazar-Ocampo, L. M. & van der Donk, W. A. In vitro activity of the nisin dehydratase NisB. Proc. Natl Acad. Sci. USA 110, 7258–7263 (2013).
Repka, L. M., Chekan, J. R., Nair, S. K. & van der Donk, W. A. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem. Rev. 117, 5457–5520 (2017).
Repka, L. M., Hetrick, K. J., Chee, S. H. & van der Donk, W. A. Characterization of leader peptide binding during catalysis by the nisin dehydratase NisB. J. Am. Chem. Soc. 140, 4200–4203 (2018).
Bothwell, I. R. et al. Characterization of glutamyl-tRNA-dependent dehydratases using nonreactive substrate mimics. Proc. Natl Acad. Sci. USA 116, 17245–17250 (2019).
Zhang, Z. & van der Donk, W. A. Nonribosomal peptide extension by a peptide amino-acyl tRNA ligase. J. Am. Chem. Soc. 141, 19625–19633 (2019).
Hudson, G. A., Zhang, Z., Tietz, J. I., Mitchell, D. A. & van der Donk, W. A. In vitro biosynthesis of the core scaffold of the thiopeptide thiomuracin. J. Am. Chem. Soc. 137, 16012–16015 (2015).
Bewley, K. D. et al. Capture of micrococcin biosynthetic intermediates reveals C-terminal processing as an obligatory step for in vivo maturation. Proc. Natl Acad. Sci. USA 113, 12450–12455 (2016).
Sikandar, A., Franz, L., Melse, O., Antes, I. & Koehnke, J. Thiazoline-specific amidohydrolase PurAH is the gatekeeper of bottromycin biosynthesis. J. Am. Chem. Soc. 141, 9748–9752 (2019).
Severinov, K. & Nair, S. K. Microcin C: biosynthesis and mechanisms of bacterial resistance. Future Microbiol. 7, 281–289 (2012).
Allali, N., Afif, H., Couturier, M. & Van Melderen, L. The highly conserved TldD and TldE proteins of Escherichia coli are involved in microcin B17 processing and in CcdA degradation. J. Bacteriol. 184, 3224–3231 (2002).
Ghilarov, D. et al. The origins of specificity in the microcin-processing protease TldD/E. Structure 25, 1549–1561.e1545 (2017).
Burkhart, B. J., Hudson, G. A., Dunbar, K. L. & Mitchell, D. A. A prevalent peptide-binding domain guides ribosomal natural product biosynthesis. Nat. Chem. Biol. 11, 564–570 (2015).
Ozaki, T. et al. Insights into the biosynthesis of dehydroalanines in goadsporin. ChemBioChem 17, 218–223 (2016).
Zhang, Q., Yu, Y., Velásquez, J. E. & van der Donk, W. A. Evolution of lanthipeptide synthetases. Proc. Natl Acad. Sci. USA 109, 18361–18366 (2012).
Zhang, M. et al. The non-canonical tetratricopeptide repeat (TPR) domain of fluorescent (FLU) mediates complex formation with glutamyl-tRNA reductase. J. Biol. Chem. 290, 17559–17565 (2015).
Perez-Riba, A. & Itzhaki, L. S. The tetratricopeptide-repeat motif is a versatile platform that enables diverse modes of molecular recognition. Curr. Opin. Struct. Biol. 54, 43–49 (2019).
Pallen, M. J., Francis, M. S. & Futterer, K. Tetratricopeptide-like repeats in type-III-secretion chaperones and regulators. FEMS Microbiol. Lett. 223, 53–60 (2003).
Das, A. K., Cohen, P. W. & Barford, D. The structure of the tetratricopeptide repeats of protein phosphatase 5: implications for TPR-mediated protein–protein interactions. EMBO J. 17, 1192–1199 (1998).
Lamb, J. R., Tugendreich, S. & Hieter, P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biochem. Sci 20, 257–259 (1995).
Fitzpatrick, P. F. Structural insights into the regulation of aromatic amino acid hydroxylation. Curr. Opin. Struct. Biol. 35, 1–6 (2015).
Fitzpatrick, P. F. Mechanism of aromatic amino acid hydroxylation. Biochemistry 42, 14083–14091 (2003).
Perdivara, I., Deterding, L. J., Przybylski, M. & Tomer, K. B. Mass spectrometric identification of oxidative modifications of tryptophan residues in proteins: chemical artifact or post-translational modification? J. Am. Soc. Mass. Spectrom. 21, 1114–1117 (2010).
Basran, J. et al. The mechanism of formation of N-formylkynurenine by heme dioxygenases. J. Am. Chem. Soc. 133, 16251–16257 (2011).
Hirose, Y. et al. Involvement of common intermediate 3-hydroxy-l-kynurenine in chromophore biosynthesis of quinomycin family antibiotics. J. Antibiot. 64, 117–122 (2011).
Todorovski, T., Fedorova, M., Hennig, L. & Hoffmann, R. Synthesis of peptides containing 5-hydroxytryptophan, oxindolylalanine, N-formylkynurenine and kynurenine. J. Pept. Sci. 17, 256–262 (2011).
Fuson, R. C. The principle of vinylogy. Chem. Rev. 16, 1–27 (1935).
Jhulki, I., Chanani, P. K., Abdelwahed, S. H. & Begley, T. P. A remarkable oxidative cascade that replaces the riboflavin C8 methyl with an amino group during roseoflavin biosynthesis. J. Am. Chem. Soc. 138, 8324–8327 (2016).
Schwarz, J., Konjik, V., Jankowitsch, F., Sandhoff, R. & Mack, M. Identification of the key enzyme of roseoflavin biosynthesis. Angew. Chem. Int. Ed. 55, 6103–6106 (2016).
Konjik, V. et al. The crystal structure of RosB: insights into the reaction mechanism of the first member of a family of flavodoxin-like enzymes. Angew. Chem. Int. Ed. 56, 1146–1151 (2017).
Kapoor, I. & Nair, S. K. Structure-guided analyses of a key enzyme involved in the biosynthesis of an antivitamin. Biochemistry 57, 5282–5288 (2018).
Ortega, M. A. et al. Structure and tRNA specificity of MibB, a lantibiotic dehydratase from Actinobacteria involved in NAI-107 biosynthesis. Cell Chem. Biol. 23, 370–380 (2016).
Sherlin, L. D. et al. Chemical and enzymatic synthesis of tRNAs for high-throughput crystallization. RNA 7, 1671–1678 (2001).
Rio, D. C., Ares, M. J., Hannon, G. J. & Nilsen, T. W. RNA: A Laboratory Manual (Cold Spring Harbor Laboratory, 2011).
Walker, S. E. & Fredrick, K. Preparation and evaluation of acylated tRNAs. Methods 44, 81–86 (2008).
Acknowledgements
This work was supported by the National Institutes of Health (R37 GM058822 to W.v.d.D., T32 GM070421 to P.N.D, F32 GM105297 to R.S., F32 GM129944 to C.P.T., and R01 GM085770 to B.S.M.). We thank M. A. Funk and K.-K. A. Wang for initial attempts to activate the bha cluster and D. A. Berthold for help in purifying GlyQS.
Author information
Authors and Affiliations
Contributions
P.N.D., H.L., R.A.S., C.P.T., and W.A.v.d.D. designed the study. P.N.D., H.L., R.A.S., C.P.T. and X.Z. performed all experiments. L.Z. acquired and interpreted the NMR data. B.S.M. provided reagents and helpful discussions; P.N.D. and W.A.v.d.D. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Seokhee Kim, A. James Link and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–34, Methods and Tables 1–5.
Rights and permissions
About this article
Cite this article
Daniels, P.N., Lee, H., Splain, R.A. et al. A biosynthetic pathway to aromatic amines that uses glycyl-tRNA as nitrogen donor. Nat. Chem. 14, 71–77 (2022). https://doi.org/10.1038/s41557-021-00802-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00802-2
This article is cited by
-
Metabolomics analysis of Dendrobium officinale tissue-cultured seedlings under red-blue composed light by using HPLC and UPLC-Q/TOF-MS
Plant Cell, Tissue and Organ Culture (PCTOC) (2024)
-
Genome mining unveils a class of ribosomal peptides with two amino termini
Nature Communications (2023)
-
Hydroxytryptophan biosynthesis by a family of heme-dependent enzymes in bacteria
Nature Chemical Biology (2023)
-
Microbial rhodoquinone biosynthesis proceeds via an atypical RquA-catalyzed amino transfer from S-adenosyl-L-methionine to ubiquinone
Communications Chemistry (2022)