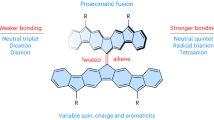
Abstract
Baird’s rule predicts that molecules with 4n π electrons should be aromatic in the triplet state, but the realization of simple ring systems with such an electronic ground state has been stymied by these molecules’ tendency to distort into structures bearing a large singlet–triplet gap. Here, we show that the elusive benzene diradical dianion can be stabilized through creation of a binucleating ligand that enforces a tightly constrained inverse sandwich structure and direct magnetic exchange coupling. Specifically, we report the compounds [K(18-crown-6)(THF)2]2[M2(BzN6-Mes)] (M = Y, Gd; BzN6-Mes = 1,3,5-tris[2′,6′-(N-mesityl)dimethanamino-4′-tert-butylphenyl]benzene), which feature a trigonal ligand that binds one trivalent metal ion on each face of a central benzene dianion. Antiferromagnetic exchange in the Gd3+ compound preferentially stabilizes the triplet state such that it becomes the molecular ground state. Single-crystal X-ray diffraction data and nucleus-independent chemical shift calculations support aromaticity, in agreement with Baird’s rule.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for the structures in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition numbers CCDC 2012304 (1-Y), 2012306 (2-Y), 2012305 (1-Gd) and 2012307 (2-Gd). Copies of data can be obtained free of charge from www.ccdc.cam.ac.uk/structures. Additional synthetic methods, nuclear magnetic resonance spectra, UV-vis-NIR spectra, single crystal X-ray diffraction data, EPR spectra, magnetism data and computational details are available in the Supplementary Information and Extended Data. Source data for Supplementary Figs. 20 and 22–31 and input files for computations are also provided as Supplementary Data 5 and 6. Source data are provided with this paper.
References
Anslyn, E. V. & Dougherty, D. A. Modern Physical Organic Chemistry (University Science Books, 2006).
Gleiter, R. Aromaticity and Other Conjugation Effects (Wiley-VCH, 2012).
Dewar, M. J. S. Aromaticity and pericyclic reactions. Angew. Chem. Int. Ed. 10, 761–776 (1971).
Fallon, K. J. et al. Exploiting excited-state aromaticity to design highly stable singlet fission materials. J. Am. Chem. Soc. 141, 13867–13876 (2019).
Randić, M. Aromaticity of polycyclic conjugated hydrocarbons. Chem. Rev. 2003, 3449–3605 (2003).
Hückel, E. Quantentheoretische Beiträge zum Benzolproblem. I. Die Elektronenkonfiguration des Benzols und verwandter Verbindungen. Z. Phys. 70, 204–286 (1931).
Breslow, R., Brown, J. & Gajewski, J. J. Antiaromaticity of cyclopropenyl anions. J. Am. Chem. Soc. 89, 4383–4390 (1967).
Heilbronner, E. Hückel molecular orbitals of Möbius-type conformations of annulenes. Tetrahedron 5, 1923–1928 (1964).
Herges, R. Topology in chemistry: designing Möbius molecules. Chem. Rev. 106, 4820–4842 (2006).
Rappaport, S. M. & Rzepa, H. S. Intrinsically chiral aromaticity. Rules incorporating linking number, twist, and writhe for higher-twist Möbius annulenes. J. Am. Chem. Soc. 130, 7613–7619 (2008).
Baird, N. C. Quantum organic photochemistry. II. Resonance and aromaticity in the lowest 3ππ* state of cyclic hydrocarbons. J. Am. Chem. Soc. 94, 4941–4948 (1972).
Ottosson, H. Exciting excited-state aromaticity. Nat. Chem. 4, 969–971 (2012).
Rosenberg, M., Dahlstrand, C., Kilså, K. & Ottosson, H. Excited state aromaticity and antiaromaticity: opportunities for photophysical and photochemical rationalizations. Chem. Rev. 114, 5379–5425 (2014).
Wan, P. & Krogh, E. Evidence for the generation of aromatic cationic systems in the excited state. Photochemical solvolysis of fluoren-9-ol. J. Chem. Soc. Chem. Commun. 1207–1208 (1985).
Ueda, M. et al. Energetics of Baird aromaticity supported by inversion of photoexcited chiral [4n]annulene derivatives. Nat. Commun. 8, 346 (2017).
Kim, J. et al. Two-electron transfer stabilized by excited-state aromatization. Nat. Commun. 10, 4983 (2019).
Kostenko, A. et al. Spectroscopic observation of the triplet diradical state of cyclobutadiene. Angew. Chem. Int. Ed. 56, 10183–10187 (2017).
Oh, J., Sung, Y. M., Hong, Y. & Kim, D. Spectroscopic diagnosis of excited-state aromaticity: capturing electronic structure and conformations upon aromaticity reversal. Acc. Chem. Res. 51, 1349–1358 (2018).
Hada, M. et al. Structural monitoring of the onset of excited-state aromaticity in a liquid crystal phase. J. Am. Chem. Soc. 139, 15792–15800 (2017).
Sung, Y. M. et al. Reversal of Hückel anti(aromaticity) in the lowest triplet states of hexaphyrins and spectroscopic evidence for Baird’s rule. Nat. Chem. 7, 418–422 (2015).
Fratev, F., Monev, V. & Janoschek, R. Ab initio study of cyclobutadiene in excited states: optimized geometries, electronic transitions and aromaticities. Tetrahedron 38, 2929–2932 (1982).
Ni, Y. et al. 3D global aromaticity in a fully conjugated diradicaloid cage at difference oxidation states. Nat. Chem. 12, 242–248 (2020).
Cha, W.-Y. et al. Bicyclic Baird-type aromaticity. Nat. Chem. 9, 1243–1248 (2017).
Valiev, R. R., Fliegl, H. & Sundholm, D. Bicycloaromaticity and Baird-type bicycloaromaticity of dithienothiophene-bridged [34]octaphyrins. Phys. Chem. Chem. Phys. 20, 17705–17713 (2018).
Falceto, A., Casanova, D., Alemany, P. & Alvarez, S. Distortions of π-coordinated arenes with anionic character. Chem. Eur. J. 20, 14674–14689 (2014).
Breslow, R., Hill, R. & Wasserman, E. Pentachlorocyclopentadienyl cation, a ground-state triplet. J. Am. Chem. Soc. 86, 5349–5350 (1964).
Wasserman, E., Hutton, R. S., Kuck, V. J. & Chandross, E. A. Dipositive ion of hexachlorobenzene, a ground-state triplet. J. Am. Chem. Soc. 96, 1965–1966 (1974).
MacDonald, M. R., Bates, J. E., Ziller, J. W., Furche, F. & Evans, W. J. Completing the series of +2 ions for the lanthanide elements: synthesis of molecular complexes of Pr2+, Gd2+, Tb2+, and Lu2+. J. Am. Chem. Soc. 135, 9857–9868 (2013).
Groom, C. R., Bruno, I. J., Lightfoot, M. P. & Ward, S. C. The Cambridge Structural Database. Acta. Cryst. 72, 171–179 (2016).
Astashkin, A. V. & Schweiger, A. Electron-spin transient nutation: a new approach to simplify the interpretation of ESR spectra. Chem. Phys. Lett. 174, 595–602 (1990).
Bleaney, B. & Bowers, K. D. Anomalous paramagnetism of copper acetate. Proc. Roy. Soc. A. 214, 451–465 (1952).
Ebata, K. et al. Planar hexasilylbenzene dianion with thermally accessible triplet state. J. Am. Chem. Soc. 120, 1335–1336 (1998).
Sekiguchi, A., Ebata, K., Kabuto, C. & Sakurai, H. Bis[(dimethoxyethane)lithium(i)] 1,2,4,5-tetrakis(trimethylsilyl)benzenide. The first 6C-8π antiaromatic benzene dianion. J. Am. Chem. Soc. 113, 7081–7082 (1991).
Demir, S., Jeon, I.-R., Long, J. R. & Harris, T. D. Radical-ligand containing single-molecule magnets. Coord. Chem. Rev. 289–290, 149–176 (2015).
Diaconescu, P. L., Arnold, P. L., Baker, T. A., Mindiola, D. J. & Cummins, C. C. Arene-bridged diuranium complexes: inverted sandwiches supported by δ backbonding. J. Am. Chem. Soc. 122, 6108–6109 (2000).
Pampaloni, G. Aromatic hydrocarbons as ligands. Recent advances in the synthesis, reactivity and the applications of bis(η6-arene) complexes. Coord. Chem. Rev. 254, 402–419 (2010).
von Ragué Schleyer, P., Maerker, C., Dransfeld, A., Jiao, H. & van Eikema Hommes, N. J. R. Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J. Am. Chem. Soc. 118, 6317–6318 (1996).
Stanger, A. Nucleus-independent chemical shifts (NICS): distance dependence and revised criteria for aromaticity and antiaromaticity. J. Org. Chem. 71, 883–893 (2006).
Stanger, A. Obtaining relative induced ring currents quantitatively from NICS. J. Org. Chem. 75, 2281–2288 (2010).
Gershoni-Poranne, R. & Stanger, A. The NICS-XY-scan: identification of local and global ring currents in multi-ring systems. Chem. Eur. J. 20, 5673–5688 (2014).
Stanger, A. Reexamination of NICSπ,zz: height dependence, off-center values, and integration. J. Phys. Chem. A 123, 3922–3927 (2019).
Kruszewski, J. & Krygowski, T. M. Definition of aromaticity basing on the harmonic oscillator model. Tetrahedron Lett. 13, 3839–3842 (1972).
Klingele, M. H. & Kersting, B. Z. Efficient medium-scale synthesis of 2-bromo-5-tert-butylbenzene-1,3-dicarbaldehyde. Z. Naturforsch. 56b, 437–439 (2001).
Lappert, M. F. & Pearce, R. Stable silylmethyl and neopentyl complexes of scandium(iii) and yttrium(iii). J. Chem. Soc. Chem. Commun. 126 (1973).
Liu, Y., Niu, F., Lian, J., Zeng, P. & Niu, H. Synthesis and properties of starburst amorphous molecules: 1,3,5-tris(1,8-naphthalimide-4-yl)benzenes. Synth. Met. 160, 2055–2060 (2010).
CrysAlisPro Software System v.1.171.39.7a (Rigaku Corporation, 2015).
SAINT and APEX 2 Software for CCD Diffractometers (Bruker Analytical X-ray Systems Inc., 2014).
Sheldrick, G. M. SADABS v.2.03 (Bruker Analytical X-ray Systems Inc., 2000).
Sheldrick, G. M. SHELXT and SHELXL (University of Göttingen, 2015).
Hassan, A. K. et al. Ultrawide band multifrequency high-field EMR technique: a methodology for increasing spectroscopic information. J. Magn. Reson. 142, 300–312 (2000).
Oyala, P. H. et al. Biophysical characterization of fluorotyrosine probes site-specifically incorporated into enzymes: E. coli ribonucleotide reductase as an example. J. Am. Chem. Soc. 138, 7951–7964 (2016).
Chilton, N. F., Anderson, R. P., Turner, L. D., Soncini, A. & Murray, K. S. PHI: a powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 34, 1164–1175 (2013).
Neese, F. The ORCA program system. WIREs Comput. Mol. Sci. 2, 73–78 (2012).
Frisch, M. J. et. al. Gaussian 09 Revision A.02 (Gaussian Inc., 2009).
Rahalkar, A. & Stanger, A. The Aroma Package, http://schulich.technion.ac.il/Amnon_Stanger.htm
Acknowledgements
This work was funded by NSF grant CHE-1800252 (C.A.G., J.R.L.), NSF grant DMR-1610226 (J.M., S.H.) and NIH grant 1R35GM126961 (D.A.M., R.D.B.). High-field EPR data were collected at the National High Magnetic Field Laboratory, which is supported by the NSF (DMR-1644779) and the State of Florida. NMR spectroscopy was collected at UC Berkeley’s NMR facility in the College of Chemistry, which is supported in part by NIH grant S10OD024998. V.V. acknowledges a postdoctoral fellowship from Fonds Wetenschappelijk Onderzoek Vlaanderen (FWO, Flemish Science Foundation) and a V435018N FWO travel grant to UC Berkeley and C.A.G. thanks the NSF Graduate Research Fellowship Program for support. In addition, we thank L. A. Berben for the use of her gloveboxes, R. G. Bergman for insightful discussions and A. Stanger for advice on the Aroma program and NICSπ,zz calculations. We also thank K. R. Meihaus for editorial assistance, N. S. Settineri for assistance with crystallography and A. B. Turkiewicz for keen observations.
Author information
Authors and Affiliations
Contributions
Synthesis, crystallography and magnetic characterization were performed by C.A.G. High-frequency CW-EPR experiments were performed and analysed by J.M. and S.H. and D-Band EPR experiments were performed and analysed by D.A.M. and R.D.B. DFT calculations and computational analysis of aromaticity were performed by V.V. and L.F.C. The manuscript was written by C.A.G. and J.R.L. and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Dongho Kim, Amnon Stanger, Dage Sundholm and Jishan Wu for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 UV-Vis-NIR spectra of [M2(BzN6-Mes)]−.
Extended Data Fig. 2 UV-Vis-NIR spectra of [M2(BzN6-Mes)]2−.
Extended Data Fig. 3 Density functional theory calculations on 2-Y.
The two singly-occupied molecular orbitals (SOMOs) obtained for 2-Y by DFT calculations are localized on the central benzene ring of the BzN6-Mes ligand.
Extended Data Fig. 4
Extended Data Fig. 5
CW-EPR spectra of 2-Y from 5 to 225 K at 371 GHz.
Extended Data Fig. 6
Double integrated absorption of the EPR spectrum of 2-Y from 5 to 225 K at 371 GHz.
Extended Data Fig. 7 Dc magnetic susceptibility measurement for 2-Gd under an applied dc magnetic field of 1000 Oe.
The black line represents a fit to the data using the listed parameters.
Extended Data Fig. 8 Dc magnetic susceptibility measurement for 2-Gd under an applied dc magnetic field of 5000 Oe.
The black line represents a fit to the data using the listed parameters.
Supplementary information
Supplementary Information
Supplementary Figs. 1–45, Tables 1–10 and Discussion
Supplementary Data 1
Single-crystal X-ray diffraction data for 1-Y.
Supplementary Data 2
Single-crystal X-ray diffraction data for 1-Gd.
Supplementary Data 3
Single-crystal X-ray diffraction data for 2-Y.
Supplementary Data 4
Single-crystal X-ray diffraction data for 2-Gd.
Supplementary Data 5
Source data for Supplementary Figs. 20 and 22–31.
Supplementary Data 6
Input files for computations.
Source data
Source Data Fig. 4
NICSπ,zz calculations for 2-Y.
Source Data Extended Data Fig. 5
CW-EPR spectrum of 2-Y from 5 to 225 K.
Source Data Extended Data Fig. 6
Double integrated absorption of the EPR spectrum of 2-Y from 5 to 225 K.
Source Data Extended Data Fig. 7
Magnetic susceptibility (d.c.) data for 2-Gd under an applied magnetic field of 1,000 Oe.
Source Data Extended Data Fig. 8
Magnetic susceptibility (d.c.) data for 2-Gd under an applied magnetic field of 5,000 Oe.
Rights and permissions
About this article
Cite this article
Gould, C.A., Marbey, J., Vieru, V. et al. Isolation of a triplet benzene dianion. Nat. Chem. 13, 1001–1005 (2021). https://doi.org/10.1038/s41557-021-00737-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00737-8
This article is cited by
-
Aromaticity rules
Nature Chemistry (2022)