Abstract
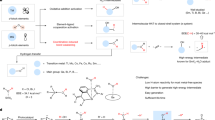
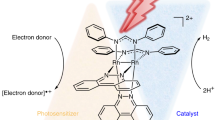
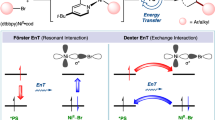
The synthesis of weak chemical bonds at or near thermodynamic potential is a fundamental challenge in chemistry, with applications ranging from catalysis to biology to energy science. Proton-coupled electron transfer using molecular hydrogen is an attractive strategy for synthesizing weak element–hydrogen bonds, but the intrinsic thermodynamics presents a challenge for reactivity. Here we describe the direct photocatalytic synthesis of extremely weak element–hydrogen bonds of metal amido and metal imido complexes, as well as organic compounds with bond dissociation free energies as low as 31 kcal mol−1. Key to this approach is the bifunctional behaviour of the chromophoric iridium hydride photocatalyst. Activation of molecular hydrogen occurs in the ground state and the resulting iridium hydride harvests visible light to enable spontaneous formation of weak chemical bonds near thermodynamic potential with no by-products. Photophysical and mechanistic studies corroborate radical-based reaction pathways and highlight the uniqueness of this photodriven approach in promoting new catalytic chemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are included with the Article and Supplementary Information. Crystallographic data for the structure of Ir5 reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2021155. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.Source data are provided with this paper.
References
Warren, J. J., Tronic, T. A. & Mayer, J. M. Thermochemistry of proton-coupled electron transfer reagents and its implications. Chem. Rev. 110, 6961–7001 (2010).
Stubbe, J., Nocera, D. G., Yee, C. S. & Chang, M. C. Y. Radical initiation in the class I ribonucleotide reductase: long-range proton-coupled electron transfer? Chem. Rev. 103, 2167–2202 (2003).
Meyer, T. J., Huynh, M. H. V. & Thorp, H. H. The possible role of proton-coupled electron transfer (PCET) in water oxidation by photosystem II. Angew. Chem. Int. Ed. 46, 5284–5304 (2007).
Bezdek, M. J., Guo, S. & Chirik, P. J. Coordination-induced weakening of ammonia, water, and hydrazine X–H bonds in a molybdenum complex. Science 354, 730–733 (2016).
Bezdek, M. J., Pappas, I. & Chirik, P. J. in Nitrogen Fixation (ed. Nishibayashi, Y.) 1–21 (Springer International Publishing, 2017).
Bezdek, M. J. & Chirik, P. J. A fresh approach to ammonia synthesis. Nature 568, 464–466 (2019).
Gentry, E. C. & Knowles, R. R. Synthetic applications of proton-coupled electron transfer. Acc. Chem. Res. 49, 1546–1556 (2016).
Chalkley, M. J. et al. Catalytic N2-to-NH3 conversion by Fe at lower driving force: a proposed role for metallocene-mediated PCET. ACS Cent. Sci. 3, 217–223 (2017).
Nicolaou, K. C., Ellery, S. P. & Chen, J. S. Samarium diiodide mediated reactions in total synthesis. Angew. Chem. Int. Ed. 48, 7140–7165 (2009).
Kolmar, S. S. & Mayer, J. M. SmI2(H2O)n reduction of electron rich enamines by proton-coupled electron transfer. J. Am. Chem. Soc. 139, 10687–10692 (2017).
Ashida, Y., Arashiba, K., Nakajima, K. & Nishibayashi, Y. Molybdenum-catalysed ammonia production with samarium diiodide and alcohols or water. Nature 568, 536–540 (2019).
Lennox, J. C., Kurtz, D. A., Huang, T. & Dempsey, J. L. Excited-state proton-coupled electron transfer: different avenues for promoting proton/electron movement with solar photons. ACS Energy Lett. 2, 1246–1256 (2017).
Pannwitz, A. & Wenger, O. S. Proton coupled electron transfer from the excited state of a ruthenium(ii) pyridylimidazole complex. Phys. Chem. Chem. Phys. 18, 11374–11382 (2016).
Martinez, K., Stash, J., Benson, K. R., Paul, J. J. & Schmehl, R. H. Direct observation of sequential electron and proton transfer in excited-state ET/PT Reactions. J. Phys. Chem. C 123, 2728–2735 (2019).
Schreier, M. R., Pfund, B., Guo, X. & Wenger, O. S. Photo-triggered hydrogen atom transfer from an iridium hydride complex to unactivated olefins. Chem. Sci. 11, 8582–8594 (2020).
Smith, D. M., Pulling, M. E. & Norton, J. R. Tin-free and catalytic radical cyclizations. J. Am. Chem. Soc. 129, 770–771 (2007).
Yao, C., Dahmen, T., Gansäuer, A. & Norton, J. Anti-Markovnikov alcohols via epoxide hydrogenation through cooperative catalysis. Science 364, 764–767 (2019).
Hu, Y. & Norton, J. R. Kinetics and thermodynamics of H–/H•/H+ transfer from a rhodium(iii) hydride. J. Am. Chem. Soc. 136, 5938–5948 (2014).
Pappas, I. & Chirik, P. J. Ammonia synthesis by hydrogenolysis of titanium–nitrogen bonds using proton coupled electron transfer. J. Am. Chem. Soc. 137, 3498–3501 (2015).
Kim, S., Zhong, H., Park, Y., Loose, F. & Chirik, P. J. Catalytic hydrogenation of a manganese(v) nitride to ammonia. J. Am. Chem. Soc. 142, 9518–9524 (2020).
Choi, J., Pulling, M. E., Smith, D. M. & Norton, J. R. Unusually weak metal−hydrogen bonds in HV(CO)4(P−P) and their effectiveness as H• donors. J. Am. Chem. Soc. 130, 4250–4252 (2008).
Choi, J., Tang, L. & Norton, J. R. Kinetics of hydrogen atom transfer from (η5-C5H5)Cr(CO)3H to various olefins: influence of olefin Structure. J. Am. Chem. Soc. 129, 234–240 (2007).
Borowski, A. F., Vendier, L., Sabo-Etienne, S., Rozycka-Sokolowska, E. & Gaudyn, A. V. Catalyzed hydrogenation of condensed three-ring arenes and their N-heteroaromatic analogues by a bis(dihydrogen) ruthenium complex. Dalton Trans. 41, 14117–14125 (2012).
Han, B., Ma, P., Cong, X., Chen, H. & Zeng, X. Chromium- and cobalt-catalyzed, regiocontrolled hydrogenation of polycyclic aromatic hydrocarbons: a combined experimental and theoretical study. J. Am. Chem. Soc. 141, 9018–9026 (2019).
Feder, H. M. & Halpern, J. Mechanism of the cobalt carbonyl-catalyzed homogeneous hydrogenation of aromatic hydrocarbons. J. Am. Chem. Soc. 97, 7186–7188 (1975).
Szostak, M., Spain, M. & Procter, D. J. Determination of the effective redox potentials of SmI2, SmBr2, SmCl2, and their complexes with water by reduction of aromatic hydrocarbons. Reduction of anthracene and stilbene by samarium(ii) iodide–water complex. J. Org. Chem. 79, 2522–2537 (2014).
Chciuk, T. V. & Flowers, R. A. Proton-coupled electron transfer in the reduction of arenes by SmI2–water complexes. J. Am. Chem. Soc. 137, 11526–11531 (2015).
Chatterjee, A. & König, B. Birch-type photoreduction of arenes and heteroarenes by sensitized electron transfer. Angew. Chem. Int. Ed. 58, 14289–14294 (2019).
Leoni, P., Landi, A. & Pasquali, M. Isolation of the first neutral chromium formyl derivatives. J. Organomet. Chem. 321, 365–369 (1987).
Bandy, J. A. et al. Decamethylrhenocene, (η5-C5Me5)2Re. J. Am. Chem. Soc. 110, 5039–5050 (1988).
Hu, Y. et al. Synthesis, electrochemistry, and reactivity of new iridium(iii) and rhodium(iii) hydrides. Organometallics 31, 5058–5064 (2012).
Deaton, J. C. et al. Excited-state switching between ligand-centered and charge transfer modulated by metal–carbon bonds in cyclopentadienyl iridium complexes. Inorg. Chem. 57, 15445–15461 (2018).
Barrett, S. M. et al. Mechanistic basis for tuning iridium hydride photochemistry from H2 evolution to hydride transfer hydrodechlorination. Chem. Sci. 11, 6442–6449 (2020).
Chalkley, M. J., Drover, M. W. & Peters, J. C. Catalytic N2-to-NH3 (or -N2H4) conversion by well-defined molecular coordination complexes. Chem. Rev. 120, 5582–5636 (2020).
Nishibayashi, Y., Iwai, S. & Hidai, M. Bimetallic system for nitrogen fixation: ruthenium-assisted protonation of coordinated N2 on tungsten with H2. Science 279, 540–542 (1998).
Reiners, M. et al. NH3 formation from N2 and H2 mediated by molecular tri-iron complexes. Nat. Chem. 12, 740–746 (2020).
Park, Y., Semproni, S. P., Zhong, H., Chirik, P. J. Synthesis, electronic structure, and reactivity of a planar four-coordinate, cobalt–imido complex. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202104320 (2021).
Gianetti, T. L., La Pierre, H. S. & Arnold, J. Group 5 imides and bis(imide)s as selective hydrogenation catalysts. Eur. J. Inorg. Chem. 2013, 3771–3783 (2013).
Iwasaki, K., Wan, K. K., Oppedisano, A., Crossley, S. W. M. & Shenvi, R. A. SImple, chemoselective hydrogenation with thermodynamic stereocontrol. J. Am.Chem. Soc. 136, 1300–1303 (2014).
Bullock, R. M. & Samsel, E. G. Hydrogen atom transfer reactions of transition-metal hydrides. Kinetics and mechanism of the hydrogenation of ɑ-cyclopropylstyrene by metal carbonyl hydrides. J. Am. Chem. Soc. 112, 6886–6898 (1990).
Gunasekara, T. et al. TEMPO-mediated catalysis of the sterically hindered hydrogen atom transfer reaction between (C5Ph5)Cr(CO)3H and a trityl radical. J. Am. Chem. Soc. 141, 1882–1886 (2019).
Kim, S., Loose, F., Bezdek, M. J., Wang, X. & Chirik, P. J. Hydrogenation of N-heteroarenes using rhodium precatalysts: reductive elimination leads to formation of multimetallic clusters. J. Am. Chem. Soc. 141, 17900–17908 (2019).
Barrett, S. M., Pitman, C. L., Walden, A. G. & Miller, A. J. M. Photoswitchable hydride transfer from iridium to 1-methylnicotinamide rationalized by thermochemical cycles. J. Am. Chem. Soc. 136, 14718–14721 (2014).
Solis, B. H. & Hammes-Schiffer, S. Substituent effects on cobalt diglyoxime catalysts for hydrogen evolution. J. Am. Chem. Soc. 133, 19036–19039 (2011).
Acknowledgements
This research was supported by the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences, Catalysis Science Program, under Award DE-SC0006498 and the Andlinger Center for Energy and the Environment (Princeton University). S.K. acknowledges a Samsung Scholarship for partial financial support. L.T. and G.D.S. acknowledge support from the Division of Chemical Sciences, Geosciences and Biosciences, Office of Basic Energy Sciences of the US DOE through Grant No. DE-SC0019370. G.D.S. is a CIFAR Fellow in the Bio-Inspired Energy Program. We are grateful to K. Conover (Princeton University) for assistance with photo-NMR experiments and L. Mendelsohn and D. Wang (Princeton University) for helpful discussions.
Author information
Authors and Affiliations
Contributions
Y.P., S.K. and P.J.C. conceived the project and designed the initial experiments. Y.P. and P.J.C. wrote the manuscript. Y.P. performed experiments regarding the synthesis and characterization of the metal complexes and the organic compounds and computational calculations. Y.P. and L.T. performed photophysical measurements under the supervision of G.D.S. Single-crystal X-ray diffraction analysis was performed by H.Z. All authors analysed the data, discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Gibbs free energy surface of the catalytic process was estimated based on thermochemical data from photophysical measurements and computational analysis.
Supplementary information
Supplementary Information
Supplementary Figs. 1–71, Note 1, Tables 1–10.
Source data
Source Data Fig. 2
Plot of data points for Fig. 2c,d.
Source Data Fig. 4
Plot of data points for Fig. 4d and its inset.
Source Data Fig. 5
Plot of data points for Fig. 5a,c and its inset.
Rights and permissions
About this article
Cite this article
Park, Y., Kim, S., Tian, L. et al. Visible light enables catalytic formation of weak chemical bonds with molecular hydrogen. Nat. Chem. 13, 969–976 (2021). https://doi.org/10.1038/s41557-021-00732-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00732-z
This article is cited by
-
Catalytic production of ammonia from dinitrogen employing molybdenum complexes bearing N-heterocyclic carbene-based PCP-type pincer ligands
Nature Synthesis (2023)
-
Ammonia synthesis by photocatalytic hydrogenation of a N2-derived molybdenum nitride
Nature Synthesis (2022)
-
Energy- and atom-efficient chemical synthesis with endergonic photocatalysis
Nature Reviews Chemistry (2022)
-
Light side of nitrogen fixation
Nature Synthesis (2022)