Abstract
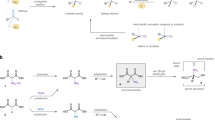
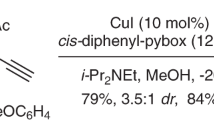
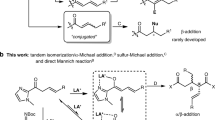
Desymmetrization of fully substituted carbons with a pair of enantiotopic functional groups is a practical strategy for the synthesis of quaternary stereocentres, as it divides the tasks of enantioselection and C−C bond formation. The use of disubstituted malonic esters as the substrate of desymmetrization is particularly attractive, given their easy and modular preparation, as well as the high synthetic values of the chiral monoester products. Here, we report that a dinuclear zinc complex with a tetradentate ligand can selectively hydrosilylate one of the carbonyls of malonic esters to give α-quaternary β-hydroxyesters, providing a promising alternative to the desymmetric hydrolysis using carboxylesterases. The asymmetric reduction features excellent enantiocontrol that can differentiate sterically similar substituents and high chemoselectivity towards the diester motif of substrates. Together with the versatile preparation of malonic ester substrates and post-reduction derivatization, the desymmetric reduction has enabled the synthesis of a diverse array of quaternary stereocentres with distinct structural features.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for 50a reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition number CCDC 2025159. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Quasdorf, K. W. & Overman, L. E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 516, 181–191 (2014).
Trost, B. M. & Jiang, C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis 369-396 (2006).
Corey, E. J. & Guzman-Perez, A. The catalytic enantioselective construction of molecules with quaternary carbon stereocenters. Angew. Chem. Int. Ed. 37, 388–401 (1998).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Das, J. P. & Marek, I. Enantioselective synthesis of all-carbon quaternary stereogenic centers in acyclic systems. Chem. Commun. 47, 4593–4623 (2011).
Feng, J., Holmes, M. & Krische, M. J. Acyclic quaternary carbon stereocenters via enantioselective transition metal catalysis. Chem. Rev. 117, 12564–12580 (2017).
Wendlandt, A. E., Vangal, P. & Jacobsen, E. N. Quaternary stereocentres via an enantioconvergent catalytic SN1 reaction. Nature 556, 447–451 (2018).
Braun, M. & Kotter, W. Titanium(iv)-catalyzed dynamic kinetic asymmetric transformation of alcohols, silyl ethers, and acetals under carbon allylation. Angew. Chem. Int. Ed. 43, 514–517 (2004).
Zhao, W., Wang, Z., Chu, B. & Sun, J. Enantioselective formation of all‐carbon quaternary stereocenters from indoles and tertiary alcohols bearing a directing group. Angew. Chem. Int. Ed. 54, 1910–1913 (2015).
Zeng, X.-P., Cao, Z.-Y., Wang, Y.-H., Zhou, F. & Zhou, J. Catalytic enantioselective desymmetrization reactions to all-carbon quaternary stereocenters. Chem. Rev. 116, 7330–7396 (2016).
Petersen, K. S. Nonenzymatic enantioselective synthesis of all-carbon quaternary centers through desymmetrization. Tetrahedron Lett. 56, 6523–6535 (2015).
de María, P. D., García-Burgos, C. A., Bargeman, G. & van Gemert, R. W. Pig liver esterase (PLE) as biocatalyst in organic synthesis: from nature to cloning and to practical applications. Synthesis 1439-1452 (2007).
García-Urdiales, E., Alfonso, I. & Gotor, V. Enantioselective enzymatic desymmetrizations in organic synthesis. Chem. Rev. 105, 313–354 (2005).
Toone, E. J., Werth, M. J. & Jones, J. B. Enzymes in organic synthesis. 47. Active-site model for interpreting and predicting the specificity of pig liver esterase. J. Am. Chem. Soc. 112, 4946–4952 (1990).
Provencher, L. & Jones, J. B. A concluding specification of the dimensions of the active site model of pig liver esterase. J. Org. Chem. 59, 2729–2732 (1994).
Björkling, F. et al. Enzyme catalysed hydrolysis of dialkylated propanedioic acid diesters, chain length dependent reversal of enantioselectivity. Tetrahedron 41, 1347–1352 (1985).
Musidlowska, A., Lange, S. & Bornscheuer, U. T. By overexpression in the yeast Pichia pastoris to enhanced enantioselectivity: new aspects in the application of pig liver esterase. Angew. Chem. Int. Ed. 40, 2851–2853 (2001).
Smith, M. E. et al. Investigation of the cosolvent effect on six isoenzymes of PLE in the enantioselective hydrolysis of selected α,α-disubstituted malonate esters. ChemCatChem 4, 472–475 (2012).
Wilent, J. & Petersen, K. S. Enantioselective desymmetrization of diesters. J. Org. Chem. 79, 2303–2307 (2014).
Karad, S. N., Panchal, H., Clarke, C., Lewis, W. & Lam, H. W. Enantioselective synthesis of chiral cyclopent-2-enones by nickel-catalyzed desymmetrization of malonate esters. Angew. Chem. Int. Ed. 57, 9122–9125 (2018).
Selmani, A. & Darses, S. Enantioenriched 1-tetralones via rhodium-catalyzed arylative cascade desymmetrization/acylation of alkynylmalonates. Org. Lett. 21, 8122–8126 (2019).
Marciniec, B. (ed.) Hydrosilylation: A Comprehensive Review on Recent Advances (Springer, 2009).
Rendler, S. & Oestreich, M. in Modern Reduction Methods (eds Andersson, P. G. & Munslow, I. J.) 183–207 (Wiley, 2008).
Díez-González, S. & Nolan, S. P. Transition metal-catalyzed hydrosilylation of carbonyl compounds and imines. A review. Org. Prep. Proced. Int. 39, 523–559 (2007).
Uvarov, V. M. & de Vekki, D. A. Recent progress in the development of catalytic systems for homogenous asymmetric hydrosilylation of ketones. J. Organomet. Chem. 923, 121415 (2020).
Mimoun, H., de Saint Laumer, J. Y., Giannini, L., Scopelliti, R. & Floriani, C. Enantioselective reduction of ketones by polymethylhydrosiloxane in the presence of chiral zinc catalysts. J. Am. Chem. Soc. 121, 6158–6166 (1999).
Mimoun, H. Selective reduction of carbonyl compounds by polymethylhydrosiloxane in the presence of metal hydride catalysts. J. Org. Chem. 64, 2582–2589 (1999).
Das, S., Möller, K., Junge, K. & Beller, M. Zinc-catalyzed chemoselective reduction of esters to alcohols. Chem. Eur. J. 17, 7414–7417 (2011).
Enthaler, S. & Wu, X.-F. (eds) Zinc Catalysis: Applications in Organic Synthesis (Wiley, 2015).
Enthaler, S. Rise of the zinc age in homogeneous catalysis? ACS Catal. 3, 150–158 (2013).
Wu, X. & Neumann, H. Zinc-catalyzed organic synthesis: C–C, C–N, C–O bond formation reactions. Adv. Synth. Catal. 354, 3141–3160 (2012).
Trost, B. M., Hung, C.-I. & Mata, G. Dinuclear metal‐prophenol catalysts: development and synthetic applications. Angew. Chem. Int. Ed. 59, 4240–4261 (2020).
Trost, B. M. & Mino, T. Desymmetrization of meso 1,3- and 1,4-diols with a dinuclear zinc asymmetric catalyst. J. Am. Chem. Soc. 125, 2410–2411 (2003).
Liu, Y., Han, S.-J., Liu, W.-B. & Stoltz, B. M. Catalytic enantioselective construction of quaternary stereocenters: assembly of key building blocks for the synthesis of biologically active molecules. Acc. Chem. Res. 48, 740–751 (2015).
Yang, Y., Cho, I., Qi, X., Liu, P. & Arnold, F. H. An enzymatic platform for the asymmetric amination of primary, secondary and tertiary C(sp3)–H bonds. Nat. Chem. 11, 987–993 (2019).
Zhang, F.-H., Zhang, F.-J., Li, M.-L., Xie, J.-H. & Zhou, Q.-L. Enantioselective hydrogenation of dialkyl ketones. Nat. Catal. 3, 621–627 (2020).
Walsh, P. J. & Kozlowski, M. C. in Fundamentals of Asymmetric Catalysis 297–329 (University Science Books, 2009).
Ushio, H. & Mikami, K. Asymmetric reduction of ortho-multisubstituted benzophenones catalyzed by diamine–Zn–diol complexes. Tetrahedron Lett. 46, 2903–2906 (2005).
Acknowledgements
We thank the University of Hong Kong for a start-up fund. We acknowledge funding support from the Laboratory for Synthetic Chemistry and Chemical Biology under the Health@InnoHK Program launched by the Innovation and Technology Commission, the Government of HKSAR. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. C.-M. Che and P. Chiu are acknowledged for their kind support and sharing of chemicals. K.-H. Low, J. Yip and B. Yan are acknowledged for X-ray crystallography, mass spectroscopy and NMR spectroscopy, respectively. We also thank L. Wu for helpful discussion.
Author information
Authors and Affiliations
Contributions
Z.H. conceived and designed the project. P.X. and Z.H. carried out the experiments, analysed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks Marcin Kwit and the other, anonymous, reviewers(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, experimental procedures, product characterization data and mechanistic studies.
Supplementary Data
Crystallographic data for compound 50a; CCDC reference 2025159.
Source data
Source Data Fig. 2c
Statistical Source Data.
Source Data Fig. 4a
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Xu, P., Huang, Z. Catalytic reductive desymmetrization of malonic esters. Nat. Chem. 13, 634–642 (2021). https://doi.org/10.1038/s41557-021-00715-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00715-0
This article is cited by
-
Catalytic desymmetrization reactions to synthesize all-carbon quaternary stereocentres
Nature Synthesis (2023)
-
Biomimetic asymmetric catalysis
Science China Chemistry (2023)
-
Diverse synthesis of α-tertiary amines and tertiary alcohols via desymmetric reduction of malonic esters
Nature Communications (2022)
-
The gains from breaking symmetry
Nature Chemistry (2021)