Abstract
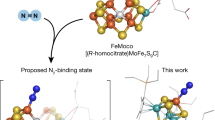
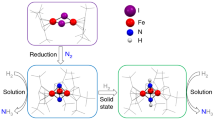
The Fe–S clusters of nitrogenases carry out the life-sustaining conversion of N2 to NH3. Although progress continues to be made in modelling the structural features of nitrogenase cofactors, no synthetic Fe–S cluster has been shown to form a well-defined coordination complex with N2. Here we report that embedding an [MoFe3S4] cluster in a protective ligand environment enables N2 binding at Fe. The bridging [MoFe3S4]2(μ-η1:η1-N2) complex thus prepared features a substantially weakened N–N bond despite the relatively high formal oxidation states of the metal centres. Substitution of one of the [MoFe3S4] cubanes with an electropositive Ti metalloradical induces additional charge transfer to the N2 ligand with generation of Fe–N multiple-bond character. Structural and spectroscopic analyses demonstrate that N2 activation is accompanied by shortened Fe–S distances and charge transfer from each Fe site, including those not directly bound to N2. These findings indicate that covalent interactions within the cluster play a critical role in N2 binding and activation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Crystallographic data for compounds were deposited in the Cambridge Structural Database under deposition numbers 2058639 (1), 2058637 (2), and 2058638 (3). Other data that support the findings of this study can be found in the article and Supplementary Information; alternatively, this information is also available upon request to the corresponding author.
References
Canfield, D. E., Glazer, A. N. & Falkowski, P. G. The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196 (2010).
Zhang, X., Ward, B. B. & Sigman, D. M. Global nitrogen cycle: critical enzymes, organisms, and processes for nitrogen budgets and dynamics. Chem. Rev. 120, 5308–5351 (2020).
Kim, J. S. & Rees, D. C. Structural models for the metal centers in the nitrogenase molybdenum–iron protein. Science 257, 1677–1682 (1992).
Einsle, O. et al. Nitrogenase MoFe-protein at 1.16 angstrom resolution: a central ligand in the FeMo-cofactor. Science 297, 1696–1700 (2002).
Lancaster, K. M. et al. X-ray emission spectroscopy evidences a central carbon in the nitrogenase iron–molybdenum cofactor. Science 334, 974–977 (2011).
Spatzal, T. et al. Evidence for interstitial carbon in nitrogenase FeMo cofactor. Science 334, 940–940 (2011).
Dos Santos, P. C. et al. Substrate interactions with the nitrogenase active site. Acc. Chem. Res. 38, 208–214 (2005).
Seefeldt, L. C., Hoffman, B. M. & Dean, D. R. Mechanism of Mo-dependent nitrogenase. Annu. Rev. Biochem. 78, 701–722 (2009).
Hoffman, B. M., Dean, D. R. & Seefeldt, L. C. Climbing nitrogenase: toward a mechanism of enzymatic nitrogen fixation. Acc. Chem. Res. 42, 609–619 (2009).
Hoffman, B. M., Lukoyanov, D., Dean, D. R. & Seefeldt, L. C. Nitrogenase: a draft mechanism. Acc. Chem. Res. 46, 587–595 (2013).
Hoffman, B. M., Lukoyanov, D., Yang, Z. Y., Dean, D. R. & Seefeldt, L. C. Mechanism of nitrogen fixation by nitrogenase: the next stage. Chem. Rev. 114, 4041–4062 (2014).
Spatzal, T., Perez, K. A., Einsle, O., Howard, J. B. & Rees, D. C. Ligand binding to the FeMo-cofactor: structures of CO-bound and reactivated nitrogenase. Science 345, 1620–1623 (2014).
Sippel, D. et al. A bound reaction intermediate sheds light on the mechanism of nitrogenase. Science 359, 1484–1489 (2018).
Kang, W., Lee, C. C., Jasniewski, A. J., Ribbe, M. W. & Hu, Y. Structural evidence for a dynamic metallocofactor during N2 reduction by Mo-nitrogenase. Science 368, 1381–1385 (2020).
Buscagan, T. M., Perez, K. A., Maggiolo, A. O., Rees, D. C. & Spatzal, T. Structural characterization of two CO molecules bound to the nitrogenase active site. Angew. Chem. Int. Ed. 60, 5704–5707 (2021).
Peters, J. W. et al. Comment on ‘Structural evidence for a dynamic metallocofactor during N2 reduction by Mo-nitrogenase’. Science 371, eabe5481 (2021).
Bergmann, J., Oksanen, E. & Ryde, U. Critical evaluation of a crystal structure of nitrogenase with bound N2 ligands. J. Biol. Inorg. Chem. 26, 341–353 (2021).
Lee, S. C. & Holm, R. H. The clusters of nitrogenase: synthetic methodology in the construction of weak-field clusters. Chem. Rev. 104, 1135–1157 (2004).
Tanifuji, K. & Ohki, Y. Metal–sulfur compounds in N2 reduction and nitrogenase-related chemistry. Chem. Rev. 120, 5194–5251 (2020).
Tanaka, K., Hozumi, Y. & Tanaka, T. Dinitrogen fixation catalyzed by the reduced species of [Fe4S4(SPh)4]2– and [Mo2Fe6S8(SPh)9]3–. Chem. Lett. 11, 1203–1206 (1982).
Hozumi, Y., Imasaka, Y., Tanaka, K. & Tanaka, T. Catalytic reduction of hydrazine to ammonia by the reduced species of [Mo2Fe6S8SPh9]3– and [Fe4S4SPh4]2–, [Mo2Fe6S8SCH2CH2OH9]3– and [Fe4S4SCH2CH2OH4]2–. Chem. Lett. 12, 897–900 (1983).
Coucouvanis, D. et al. The catalytic reduction of hydrazine to ammonia by the MoFe3S4 cubanes and implications regarding the function of nitrogenase—evidence for direct involvement of the molybdenum atom in substrate reduction. J. Am. Chem. Soc. 115, 12193–12194 (1993).
Malinak, S. M., Demadis, K. D. & Coucouvanis, D. Catalytic reduction of hydrazine to ammonia by the VFe3S4 cubanes—further evidence for the direct involvement of the heterometal in the reduction of nitrogenase substrates and possible relevance to the vanadium nitrogenases. J. Am. Chem. Soc. 117, 3126–3133 (1995).
Malinak, S. M., Simeonov, A. M., Mosier, P. E., McKenna, C. E. & Coucouvanis, D. Catalytic reduction of cis-dimethyldiazene by the [MoFe3S4]3+ clusters. The four-electron reduction of a N=N bond by a nitrogenase-relevant cluster and implications for the function of nitrogenase. J. Am. Chem. Soc. 119, 1662–1667 (1997).
Vela, J., Stoian, S., Flaschenriem, C. J., Münck, E. & Holland, P. L. A sulfido-bridged diiron(ii) compound and its reactions with nitrogenase-relevant substrates. J. Am. Chem. Soc. 126, 4522–4523 (2004).
Banerjee, A. et al. Photochemical nitrogen conversion to ammonia in ambient conditions with FeMoS-chalcogels. J. Am. Chem. Soc. 137, 2030–2034 (2015).
DeRosha, D. E. et al. Planar three-coordinate iron sulfide in a synthetic [4Fe-3S] cluster with biomimetic reactivity. Nat. Chem. 11, 1019–1025 (2019).
Thorneley, R. N. F. & Lowe, D. J. in Metal Ions in Biology, Vol. 7 (ed Spiro, T. G.) 221–284 (Wiley, 1985).
Seefeldt, L. C. et al. Reduction of substrates by nitrogenases. Chem. Rev. 120, 5082–5106 (2020).
Venkateswara Rao, P. & Holm, R. H. Synthetic analogues of the active sites of iron-sulfur proteins. Chem. Rev. 104, 527–559 (2004).
Holm, R. H. & Lo, W. Structural conversions of synthetic and protein-bound iron–sulfur clusters. Chem. Rev. 116, 13685–13713 (2016).
Deng, L. & Holm, R. H. Stabilization of fully reduced iron–sulfur clusters by carbene ligation: the [FenSn]0 oxidation levels (n = 4, 8). J. Am. Chem. Soc. 130, 9878–9886 (2008).
Brown, A. C. & Suess, D. L. M. Controlling substrate binding to Fe4S4 clusters through remote steric effects. Inorg. Chem. 58, 5273–5280 (2019).
Komuro, T., Kawaguchi, H., Lang, J. P., Nagasawa, T. & Tatsumi, K. [MoFe3S4]3+ and [MoFe3S4]2+ cubane clusters containing a pentamethylcyclopentadienyl molybdenum moiety. J. Organomet. Chem. 692, 1–9 (2007).
Singh, D., Buratto, W. R., Torres, J. F. & Murray, L. J. Activation of dinitrogen by polynuclear metal complexes. Chem. Rev. 120, 5517–5581 (2020).
Ohki, Y. et al. N2 activation on a molybdenum–titanium–sulfur cluster. Nat. Commun. 9, 3200 (2018).
Mori, H., Seino, H., Hidai, M. & Mizobe, Y. Isolation of a cubane-type metal sulfido cluster with a molecular nitrogen ligand. Angew. Chem. Int. Ed. 46, 5431–5434 (2007).
Liu, J. et al. Nitrogenase-mimic iron-containing chalcogels for photochemical reduction of dinitrogen to ammonia. Proc. Natl Acad. Sci. USA 113, 5530–5535 (2016).
Heim, H. C., Bernhardt, T. M., Lang, S. M., Barnett, R. N. & Landman, U. Interaction of iron–sulfur clusters with N2: biomimetic systems in the gas phase. J. Phys. Chem. C 120, 12549–12558 (2016).
Takaoka, A., Mankad, N. P. & Peters, J. C. Dinitrogen complexes of sulfur-ligated iron. J. Am. Chem. Soc. 133, 8440–8443 (2011).
Creutz, S. E. & Peters, J. C. Diiron bridged-thiolate complexes that bind N2 at the FeiiFeii, FeiiFei and FeiFei redox states. J. Am. Chem. Soc. 137, 7310–7313 (2015).
Coric, I., Mercado, B. Q., Bill, E., Vinyard, D. J. & Holland, P. L. Binding of dinitrogen to an iron–sulfur–carbon site. Nature 526, 96–99 (2015).
Gu, N. N. X., Oyala, P. H. & Peters, J. C. An S = ½ iron complex featuring N2, thiolate, and hydride ligands: reductive elimination of H2 and relevant thermochemical Fe–H parameters. J. Am. Chem. Soc. 140, 6374–6382 (2018).
Smith, J. M. et al. Studies of low-coordinate iron dinitrogen complexes. J. Am. Chem. Soc. 128, 756–769 (2006).
Chomitz, W. A. & Arnold, J. Transition metal dinitrogen complexes supported by a versatile monoanionic [N2P2] ligand. Chem. Commun. 45, 4797–4799 (2007).
McSkimming, A. & Harman, W. H. A terminal N2 complex of high-spin iron(i) in a weak, trigonal ligand field. J. Am. Chem. Soc. 137, 8940–8943 (2015).
Suzuki, T. et al. N2 activation by an iron complex with a strong electron-donating iminophosphorane ligand. Inorg. Chem. 54, 9271–9281 (2015).
Geri, J. B., Shanahan, J. P. & Szymczak, N. K. Testing the push–pull hypothesis: Lewis acid augmented N2 activation at iron. J. Am. Chem. Soc. 139, 5952–5956 (2017).
Horacek, M. et al. Bis[η5-tetramethyl(trimethylsilyl)cyclopentadienyl]titanium(ii) and its π-complexes with bis(trimethylsilyl)acetylene and ethylene. Organometallics 18, 3572–3578 (1999).
Lee, Y., Mankad, N. P. & Peters, J. C. Triggering N2 uptake via redox-induced expulsion of coordinated NH3 and N2 silylation at trigonal bipyramidal iron. Nat. Chem. 2, 558–565 (2010).
Moret, M. E. & Peters, J. C. N2 functionalization at iron metallaboratranes. J. Am. Chem. Soc. 133, 18118–18121 (2011).
Rittle, J. & Peters, J. C. Fe–N2/CO complexes that model a possible role for the interstitial C atom of FeMo-cofactor (FeMoco). Proc. Natl Acad. Sci. USA 110, 15898–15903 (2013).
Ung, G. & Peters, J. C. Low-temperature N2 binding to two-coordinate L2Fe0 enables reductive trapping of L2FeN2– and NH3 generation. Angew. Chem. Int. Ed. 54, 532–535 (2015).
Piascik, A. D. et al. Cationic silyldiazenido complexes of the Fe(diphosphine)2(N2) platform: structural and electronic models for an elusive first intermediate in N2 fixation. Chem. Commun. 53, 7657–7660 (2017).
Saouma, C. T. & Peters, J. C. M≡E and M=E complexes of iron and cobalt that emphasize three-fold symmetry (E = O, N, NR). Coord. Chem. Rev. 255, 920–937 (2011).
Cook, M. & Karplus, M. Electronic-structure of the MoFe3S4(SH)63– ion. J. Am. Chem. Soc. 107, 257–259 (1985).
Bjornsson, R. et al. Identification of a spin-coupled Mo(iii) in the nitrogenase iron–molybdenum cofactor. Chem. Sci. 5, 3096–3103 (2014).
Kowalska, J. K. et al. X-ray magnetic circular dichroism spectroscopy applied to nitrogenase and related models: experimental evidence for a spin-coupled molybdenum(iii) center. Angew. Chem. Int. Ed. 58, 9373–9377 (2019).
Noodleman, L., Peng, C. Y., Case, D. A. & Mouesca, J. M. Orbital interactions, electron delocalization and spin coupling in iron–sulfur clusters. Coord. Chem. Rev. 144, 199–244 (1995).
Holland, P. L. Metal–dioxygen and metal–dinitrogen complexes: where are the electrons? Dalton Trans. 39, 5415–5425 (2010).
Tan, L. L., Holm, R. H. & Lee, S. C. Structural analysis of cubane-type iron clusters. Polyhedron 58, 206–217 (2013).
Pandelia, M. E., Lanz, N. D., Booker, S. J. & Krebs, C. Mössbauer spectroscopy of Fe/S proteins. Biochim. Biophys. Acta Mol. Cell Res. 1853, 1395–1405 (2015).
Zhang, Y. G. & Holm, R. H. Synthesis of a molecular Mo2Fe6S9 cluster with the topology of the P–N cluster of nitrogenase by rearrangement of an edge-bridged Mo2Fe6S8 double cubane. J. Am. Chem. Soc. 125, 3910–3920 (2003).
Xi, B. & Holm, R. H. The [MoFe3S4]2+ oxidation state: synthesis, substitution reactions, and structures of phosphine-ligated cubane-type clusters with the S = 2 ground state. Inorg. Chem. 50, 6280–6288 (2011).
Zhang, Y. G., Zuo, J. L., Zhou, H. C. & Holm, R. H. Rearrangement of symmetrical dicubane clusters into topological analogues of the P cluster of nitrogenase: nature’s choice? J. Am. Chem. Soc. 124, 14292–14293 (2002).
Powers, T. M. & Betley, T. A. Testing the polynuclear hypothesis: multielectron reduction of small molecules by triiron reaction sites. J. Am. Chem. Soc. 135, 12289–12296 (2013).
Lukoyanov, D. A. et al. Electron redistribution within the nitrogenase active site FeMo-cofactor during reductive elimination of H2 to achieve N≡N triple-bond activation. J. Am. Chem. Soc. 142, 21679–21690 (2020).
Bantreil, X. & Nolan, S. P. Synthesis of N-heterocyclic carbene ligands and derived ruthenium olefin metathesis catalysts. Nat. Protoc. 6, 69–77 (2011).
Horacek, M., Polasek, M., Kupfer, V., Thewalt, U. & Mach, K. Syntheses and crystal structures of dichlorobis[tetramethyl(phenyl)cyclopentadienyl]titanium(iv) and chlorobis[tetramethyl(phenyl)cyclopentadienyl]-titanium(iii). Collect. Czech Chem. C 64, 61–72 (1999).
Hanna, T. E., Lobkovsky, E. & Chirik, P. J. Mono(dinitrogen) and carbon monoxide adducts of bis(cyclopentadienyl) titanium sandwiches. J. Am. Chem. Soc. 128, 6018–6019 (2006).
MestReNova v.12.0, Mestrelab Research, www.mestrelab.com (2017).
Schubert, E. M. Utilizing the Evans method with a superconducting NMR spectrometer in the undergraduate laboratory. J. Chem. Educ. 69, 62 (1992).
Bain, G. A. & Berry, J. F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 85, 532–536 (2008).
Prisecaru, I. WMOSS4 Mössbauer spectral analysis software, www.wmoss.org (2016).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
SADABS, v.2014/5 (Bruker AXS, 2001).
Krause, L., Herbst-Irmer, R., Sheldrick, G. M. & Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 48, 3–10 (2015).
Sheldrick, G. M. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. Crystal structure refinement with SHELXL. Acta Crystallogr. C 71, 3–8 (2015).
Müller, P. Practical suggestions for better crystal structures. Crystallogr. Rev. 15, 57–83 (2009).
Acknowledgements
We thank N. B. Thompson for assistance with Mössbauer spectroscopic experiments and P. Müller for assistance with X-ray crystallographic experiments. We acknowledge the MIT Research Support Committee Fund for financial support of this work.
Author information
Authors and Affiliations
Contributions
A.M. performed the experiments. A.M. and D.L.M.S. designed the research, analysed the data, and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Tables 1–6, discussion.
Crystallographic data 1
CIF file for compound 1
Crystallographic data 2
CIF file for compound 2
Crystallographic data 3
CIF file for compound 3
Rights and permissions
About this article
Cite this article
McSkimming, A., Suess, D.L.M. Dinitrogen binding and activation at a molybdenum–iron–sulfur cluster. Nat. Chem. 13, 666–670 (2021). https://doi.org/10.1038/s41557-021-00701-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00701-6
This article is cited by
-
Catalytic nitrogen fixation using visible light energy
Nature Communications (2022)
-
Taming phosphorus mononitride
Nature Chemistry (2022)
-
Nitrogen reduction by the Fe sites of synthetic [Mo3S4Fe] cubes
Nature (2022)
-
Synthetic molecular cluster hints at mechanism of nitrogen fixation
Nature (2022)
-
Pinning growth of TiN films toward porous Ti matrix
Journal of Materials Science (2022)