Abstract
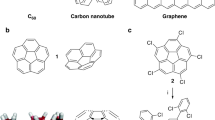
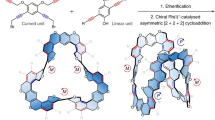
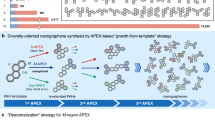
Aromatic hydrocarbon belts (AHCBs) have fascinated scientists for over half a century because of their aesthetically appealing structures and potential applications in the field of carbon nanotechnology. One of the enduring challenges in synthesizing AHCBs is how do we cope with the build-up of energy in the highly strained structures during their synthesis? Successful preparations of AHCBs offer the prospect of providing well-defined templates for the growth of uniform single-walled carbon nanotubes—a long-standing interest in nanocarbon science. In this Review, we revisit the protracted historical background involving the rational design and synthesis of AHCBs and highlight some of the more recent breakthroughs, with emphasis being placed on the different strategies that have been used for building up curved and fused benzenoid rings into molecular belts. We also discuss the scientific challenges in this fledgling field and provide some pointers as to what could transpire in years to come.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
References
Diederich, F. et al. All-carbon molecules: Evidence for the generation of cyclo[18]carbon from a stable organic precursor. Science 245, 1088–1090 (1989).
Stoddart, J. F. All-carbon compounds. Towards the cyclo[n]carbons. Nature 342, 482–483 (1989).
Stoddart, J. F. The third allotropic form of carbon. Angew. Chem. Int. Ed. Engl. 30, 70–71 (1991).
Petrukhina, M. A. & Scott, L. T. Fragments of Fullerenes and Carbon Nanotubes (Wiley, 2011).
Siegel, J. S. Allotropy by design–Carbon nanohoops. Science 356, 135–136 (2017).
Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: Buckminsterfullerene. Nature 318, 162–163 (1985).
Smalley, R. E. Discovering the fullerenes (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 36, 1594–1601 (1997).
Kroto, H. Symmetry, space, stars, and C60 (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 36, 1578–1593 (1997).
Curl, R. F. Dawn of the fullerenes: conjecture and experiment (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 36, 1566–1576 (1997).
Iijima, S. Helical microtubules of graphitic carbon. Nature 354, 56–58 (1991).
Heilbronner, E. Molecular orbitals in homologen reihen mehrkerniger aromatischer kohlenwasserstoffe: I. Die eigenwerte von LCAO-MO’s in homologen reihen. Helv. Chim. Acta 37, 921–935 (1954).
Vögtle, F. Cyclophanes II. Concluding remarks. Top. Curr.Chem. 115, 157–159 (1983).
Kohnke, F. H., Slawin, A. M. Z., Stoddart, J. F. & Williams, D. J. Molecular belts and collars in the making: A hexaepoxyoctacosahydro[12]cyclacene derivative. Angew. Chem. Int. Ed. Engl. 26, 892–894 (1987).
Ashton, P. R. et al. Towards the making of [12]collarene. Angew. Chem. Int. Ed. Engl. 27, 966–969 (1988).
Cory, R. M., McPhail, C. L., Dikmans, A. J. & Vittal, J. J. Macrocyclic cyclophane belts via double Diels–Alder cycloadditions: Macroannulation of bisdienes by bisdienophiles. Synthesis of a key precursor to an [8]cyclacene. Tetrahedron Lett. 37, 1983–1986 (1996).
Cory, R. M. & McPhail, C. L. Transformations of a macrocyclic cyclophane belt into advanced [8]cyclacene and [8]cyclacene triquinone precursors. Tetrahedron Lett. 37, 1987–1990 (1996).
Godt, A., Enkelmann, V. & Schlüter, A. D. Double-stranded molecules: a [6]beltene derivative and the corresponding open-chain polymer. Angew. Chem. Int. Ed. Engl. 28, 1680–1682 (1989).
Kintzel, O., Luger, P., Weber, M. & Schlüter, A. D. Ring-chain equilibrium between an [18]cyclacene derivative and a ladder oligomer. Eur. J. Org. Chem. 1998, 99–105 (1998).
Jasti, R., Bhattacharjee, J., Neaton, J. B. & Bertozzi, C. R. Synthesis, characterization, and theory of [9]-, [12]-, and [18]cycloparaphenylene: Carbon nanohoop structures. J. Am. Chem. Soc. 130, 17646–17647 (2008).
Povie, G., Segawa, Y., Nishihara, T., Miyauchi, Y. & Itami, K. Synthesis of a carbon nanobelt. Science 356, 172–175 (2017).
Cheung, K. Y. et al. Synthesis of armchair and chiral carbon nanobelts. Chem 5, 838–847 (2019).
Shi, T.-H., Guo, Q.-H., Tong, S. & Wang, M.-X. Toward the synthesis of a highly strained hydrocarbon belt. J. Am. Chem. Soc. 142, 4576–4580 (2020).
Sisto, T. J., Zakharov, L. N., White, B. M. & Jasti, R. Towards π-extended cycloparaphenylenes as seeds for CNT growth: investigating strain relieving ring-openings and rearrangements. Chem. Sci. 7, 3681–3688 (2016).
Segawa, Y., Ito, H. & Itami, K. Structurally uniform and atomically precise carbon nanostructures. Nat. Rev. Mater 1, 15002 (2016).
Saito, R., Fujita, M., Dresselhaus, G. & Dresselhaus, M. S. Electronic structure of chiral graphene tubules. Appl. Phys. Lett. 60, 2204–2206 (1992).
Liu, B., Wu, F., Gui, H., Zheng, M. & Zhou, C. Chirality-controlled synthesis and applications of single-wall carbon nanotubes. ACS Nano 11, 31–53 (2017).
Harris, P. J. F. Carbon Nanotubes and Related Structures: New Materials for the Twenty-First Century (Cambridge Univ. Press, 2003).
Dresselhaus, M. S., Dresselhaus, G. & Avouris, P. Carbon Nanotubes: Synthesis, Structure, Properties, and Applications (Springer, 2001).
Shi, T.-H., Tong, S. & Wang, M.-X. Construction of hydrocarbon nanobelts. Angew. Chem. Int. Ed. 59, 7700–7705 (2020).
Xia, Z., Pun, S. H., Chen, H. & Miao, Q. Synthesis of zigzag carbon nanobelts through Scholl reactions. Angew. Chem. Int. Ed. https://doi.org/10.1002/ange.202100343 (2021).
Nakamura, E., Tahara, K., Matsuo, Y. & Sawamura, M. Synthesis, structure, and aromaticity of a hoop-shaped cyclic benzenoid [10]cyclophenacene. J. Am. Chem. Soc. 125, 2834–2835 (2003).
Matsuo, Y., Tahara, K., Sawamura, M. & Nakamura, E. Creation of hoop- and bowl-shaped benzenoid systems by selective detraction of [60]fullerene conjugation. [10]Cyclophenacene and fused corannulene derivatives. J. Am. Chem. Soc. 126, 8725–8734 (2004).
Matsuo, Y., Tahara, K. & Nakamura, E. Synthesis and electrochemistry of double-decker buckyferrocenes. J. Am. Chem. Soc. 128, 7154–7155 (2006).
Matsuo, Y., Tahara, K., Morita, K., Matsuo, K. & Nakamura, E. Regioselective eightfold and tenfold additions of a pyridine-modified organocopper reagent to [60]fullerene. Angew. Chem. Int. Ed. 46, 2844–2847 (2007).
Li, Y., Xu, D. & Gan, L. Selective multiamination of C70 leading to curved π systems with 60, 58, 56, and 50 π electrons. Angew. Chem. Int. Ed. 55, 2483–2487 (2016).
Schulz, F. et al. Exploring a route to cyclic acenes by on-surface synthesis. Angew. Chem. Int. Ed. 58, 9038–9042 (2019).
Povie, G., Segawa, Y., Nishihara, T., Miyauchi, Y. & Itami, K. Synthesis and size-dependent properties of [12], [16], and [24]carbon nanobelts. J. Am. Chem. Soc. 140, 10054–10059 (2018).
Cory, R. M. & McPhail, C. L.Fascinating Stops on the Way to Cyclacenes and Cyclacene Quinones: A tour guide to synthetic progress to date. In Advances in Theoretically Interesting Molecules Vol. 4 (ed. Thummel, R. P.) 53–80 (JAI Press, 1998).
Iyoda, M., Kuwatani, Y., Nishinaga, T., Takase, M. & Nishiuchi, T. Conjugated molecular belts based on 3D benzannulene systems. In Fragments of Fullerenes and Carbon Nanotubes (eds Petrukhina, M. A. & Scott, L. T.) 311–342 (2011).
Tahara, K. & Tobe, Y. Molecular loops and belts. Chem. Rev. 106, 5274–5290 (2006).
Eisenberg, D., Shenhar, R. & Rabinovitz, M. Synthetic approaches to aromatic belts: building up strain in macrocyclic polyarenes. Chem. Soc. Rev. 39, 2879–2890 (2010).
Segawa, Y., Yagi, A., Matsui, K. & Itami, K. Design and synthesis of carbon nanotube segments. Angew. Chem. Int. Ed. 55, 5136–5158 (2016).
Yao, T., Yu, H., Vermeij, R. J. & Bodwell, G. J. Nonplanar aromatic compounds. Part 10: a strategy for the synthesis of aromatic belts—all wrapped up or down the tubes? Pure Appl. Chem. 80, 533–546 (2008).
Türker, L. & Gümüş, S. Cyclacenes. J. Mol. Struct. THEOCHEM 685, 1–33 (2004).
Gleiter, R., Esser, B. & Kornmayer, S. C. Cyclacenes: Hoop-shaped systems composed of conjugated rings. Acc. Chem. Res. 42, 1108–1116 (2009).
Shi, T.-H. & Wang, M.-X. Zigzag hydrocarbon belts. CCS Chem 2, 916–931 (2020).
Cheung, K. Y., Segawa, Y. & Itami, K. Synthetic strategies of carbon nanobelts and related belt-shaped polycyclic aromatic hydrocarbons. Chem. Eur. J. 26, 14791–14801 (2020).
Chen, H. & Miao, Q. Recent advances and attempts in synthesis of conjugated nanobelts. J. Phys. Org. Chem. 33, e4145 (2020).
Hermann, M., Wassy, D. & Esser, B. Conjugated nanohoops incorporating donor-, acceptor-, hetero- or polycyclic aromatics. Angew. Chem. Int. Ed. https://doi.org/10.1002/anie.202007024 (2021).
Scott, L. T. Conjugated belts and nanorings with radially oriented p orbitals. Angew. Chem. Int. Ed. 42, 4133–4135 (2003).
Segawa, Y., Yagi, A., Ito, H. & Itami, K. A theoretical study on the strain energy of carbon nanobelts. Org. Lett. 18, 1430–1433 (2016).
Golder, M. R. & Jasti, R. Syntheses of the smallest carbon nanohoops and the emergence of unique physical phenomena. Acc. Chem. Res. 48, 557–566 (2015).
Hitosugi, S., Nakanishi, W., Yamasaki, T. & Isobe, H. Bottom-up synthesis of finite models of helical (n,m)-single-wall carbon nanotubes. Nat. Commun. 2, 492 (2011).
Iwamoto, T., Kayahara, E., Yasuda, N., Suzuki, T. & Yamago, S. Synthesis, characterization, and properties of [4]cyclo-2,7-pyrenylene: Effects of cyclic structure on the electronic properties of pyrene oligomers. Angew. Chem. Int. Ed. 53, 6430–6434 (2014).
Huang, Q. et al. Photoconductive curved-nanographene/fullerene supramolecular heterojunctions. Angew. Chem. Int. Ed. 58, 6244–6249 (2019).
Herges, R. Fully Conjugated beltenes (belt-like and tubular aromatics). In Modern Cyclophane Chemistry (eds Gleiter, R. & Hopf, H.) 337–358 (2005).
Kawase, T., Darabi, H. R. & Oda, M. Cyclic [6]- and [8]paraphenylacetylenes. Angew. Chem. Int. Ed. Engl. 35, 2664–2666 (1996).
Kawase, T., Tanaka, K., Shiono, N., Seirai, Y. & Oda, M. Onion-type complexation based on carbon nanorings and a buckminsterfullerene. Angew. Chem. Int. Ed. 43, 1722–1724 (2004).
Segawa, Y., Levine, D. R. & Itami, K. Topologically unique molecular nanocarbons. Acc. Chem. Res. 52, 2760–2767 (2019).
Choi, H. S. & Kim, K. S. Structures, magnetic properties, and aromaticity of cyclacenes. Angew. Chem. Int. Ed. 38, 2256–2258 (1999).
Chen, Z. et al. Open-shell singlet character of cyclacenes and short zigzag nanotubes. Org. Lett. 9, 5449–5452 (2007).
Clar, E. The Aromatic Sextet (Wiley, 1972).
Clar, E. Polycyclic Hydrocarbons. (Academic Press, 1964).
Deichmann, M., Nather, C. & Herges, R. Pyrolysis of a tubular aromatic compound. Org. Lett. 5, 1269–1271 (2003).
Schaller., G. R. & Herges, R. Aromatic belts as sections of nanotubes. In Fragments of Fullerenes and Carbon Nanotubes (eds Petrukhina, M. A. & Scott, L. T.) 259–289 (2011).
Vögtle, F., Schröder, A. & Karbach, D. Strategy for the synthesis of tube-shaped molecules. Angew. Chem. Int. Ed. Engl. 30, 575–577 (1991).
Golling, F. E., Quernheim, M., Wagner, M., Nishiuchi, T. & Müllen, K. Concise synthesis of 3D π-extended polyphenylene cylinders. Angew. Chem. Int. Ed. 53, 1525–1528 (2014).
Yagi, A., Segawa, Y. & Itami, K. Armchair and chiral carbon nanobelts: Scholl reaction in strained nanorings. Chem 5, 746–748 (2019).
Sekiguchi, R. et al. Preparation of a cyclic polyphenylene array for a zigzag-type carbon nanotube segment. J. Org. Chem. 80, 5092–5110 (2015).
Korich, A. L., McBee, I. A., Bennion, J. C., Gifford, J. I. & Hughes, T. S. Synthesis and photophysical properties of biphenyl and terphenyl arylene−ethynylene macrocycles. J. Org. Chem. 79, 1594–1610 (2014).
Merner, B. L., Dawe, L. N. & Bodwell, G. J. 1,1,8,8-Tetramethyl[8](2,11)teropyrenophane: half of an aromatic belt and a segment of an (8,8) single-walled carbon nanotube. Angew. Chem. Int. Ed. 48, 5487–5491 (2009).
Stoddart, J. F. Unnatural product synthesis. Nature 334, 10–11 (1988).
Myśliwiec, D. & Stępień, M. The fold-in approach to bowl-shaped aromatic compounds: Synthesis of chrysaoroles. Angew. Chem. Int. Ed. 52, 1713–1717 (2013).
Yang, Y., Yuan, L., Shan, B., Liu, Z. & Miao, Q. Twisted polycyclic arenes from tetranaphthyldiphenylbenzenes by controlling the Scholl reaction with substituents. Chem. Eur. J. 22, 18620–18627 (2016).
Pedersen, C. J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 89, 7017–7036 (1967).
Pedersen, C. J. The discovery of crown ethers (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 27, 1021–1027 (1988).
Gutsche, C. D., Dhawan, B., No, K. H. & Muthukrishnan, R. Calixarenes. 4. The synthesis, characterization, and properties of the calixarenes from p-tert-butylphenol. J. Am. Chem. Soc. 103, 3782–3792 (1981).
Gutsche, C. D. Calixarenes. Acc. Chem. Res. 16, 161–170 (1983).
Freeman, W. A., Mock, W. L. & Shih, N. Y. Cucurbituril. J. Am. Chem. Soc. 103, 7367–7368 (1981).
Lagona, J., Mukhopadhyay, P., Chakrabarti, S. & Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Ed. 44, 4844–4870 (2005).
Ogoshi, T., Kanai, S., Fujinami, S., Yamagishi, T. A. & Nakamoto, Y. para-Bridged symmetrical pillar[5]arenes: their Lewis acid catalyzed synthesis and host–guest property. J. Am. Chem. Soc. 130, 5022–5023 (2008).
Guo, Q.-H., Fu, Z.-D., Zhao, L. & Wang, M.-X. Synthesis, structure, and properties of O6-Corona[3]arene[3]tetrazines. Angew. Chem. Int. Ed. 53, 13548–13552 (2014).
Wang, M.-X. Coronarenes: Recent advances and perspectives on macrocyclic and supramolecular chemistry. Sci. China Chem. 61, 993–1003 (2018).
San-Fabián, E., Pérez-Guardiola, A., Moral, M., Pérez-Jiménez, A. J. & Sancho-García, J. C. Theoretical study of strained carbon-based nanobelts: structural, energetic, electronic, and magnetic properties of [n]cyclacenes. In Advanced Magnetic and Optical Materials (eds Tiwari, A., Iyer, P. K., Kumar, V., & Swart, H.) 165–183 (Scrivener, 2016).
Houk, K. N., Lee, P. S. & Nendel, M. Polyacene and cyclacene geometries and electronic structures: bond equalization, vanishing band gaps, and triplet ground states contrast with polyacetylene. J. Org. Chem. 66, 5517–5521 (2001).
Battaglia, S. et al. A theoretical study on cyclacenes: analytical tight-binding approach. Int. J. Quantum Chem. 118, e25569 (2018).
Sadowsky, D., McNeill, K. & Cramer, C. J. Electronic structures of [n]-cyclacenes (n = 6–12) and short, hydrogen-capped, carbon nanotubes. Faraday Discuss. 145, 507–521 (2010).
Wu, C. S., Lee, P. Y. & Chai, J. D. Electronic properties of cyclacenes from TAO-DFT. Sci. Rep. 6, 37249 (2016).
Battaglia, S., Faginas-Lago, N., Andrae, D., Evangelisti, S. & Leininger, T. Increasing radical character of large [n]cyclacenes unveiled by wave function theory. J. Phys. Chem. A 121, 3746–3756 (2017).
Türker, L. Cryptoannulenic behavior of cyclacenes. Polycyclic Aromat. Compd. 4, 191–197 (1994).
Loh, K. P., Yang, S. W., Soon, J. M., Zhang, H. & Wu, P. Ab initio studies of borazine and benzene cyclacenes and their fluoro-substituted derivatives. J. Phys. Chem. A 107, 5555–5560 (2003).
Li, Q., Xu, H.-L. & Su, Z.-M. NICS values scan in three-dimensional space of the hoop-shaped π-conjugated molecules [6]8cyclacene and [16]trannulene. New J. Chem. 42, 1987–1994 (2018).
Diels, O. & Alder, K. Synthesen in der hydroaromatischen reihe. Justus Liebigs Ann. Chem 460, 98–122 (1928).
Standera, M. & Schlüter, D. Toward fully unsaturated double-stranded cycles. In Fragments of Fullerenes and Carbon Nanotubes (eds Petrukhina, M. A. & Scott, L. T.) 343–366 (2011).
Matsui, K., Fushimi, M., Segawa, Y. & Itami, K. Synthesis, structure, and reactivity of a cylinder-shaped cyclo[12]orthophenylene[6]ethynylene: toward the synthesis of zigzag carbon nanobelts. Org. Lett. 18, 5352–5355 (2016).
Cheung, K. Y., Watanabe, K., Segawa, Y. & Itami, K. Synthesis of a zigzag carbon nanobelt. Nat. Chem. 13, 255–259 (2021).
Han, Y., Dong, S., Shao, J., Fan, W. & Chi, C. Synthesis of a sidewall fragment of a (12,0) carbon nanotube. Angew. Chem. Int. Ed. 60, 2658–2662 (2021).
Elmosalamy, M. A. F., Moody, G. J., Thomas, J. D. R., Kohnke, F. A. & Stoddart, J. F. Studies on two epoxyoctacosahydro[12]cyclacene derivatives as sensor coatings on quartz piezoelectric crystals for detecting aromatic vapours. Anal. Proc. 26, 12–15 (1989).
Ashton, P. R., Isaacs, N. S., Kohnke, F. H., Mathias, J. P. & Stoddart, J. F. Stereoregular oligomerization by repetitive Diels–Alder reactions. Angew. Chem. Int. Ed. Engl. 28, 1258–1261 (1989).
Ashton, P. R., Isaacs, N. S., Kohnke, F. H., D’Alcontres, G. S. & Stoddart, J. F. Trinacrene–a product of structure-directed synthesis. Angew. Chem. Int. Ed. Engl. 28, 1261–1263 (1989).
Ashton, P. R. et al. Molecular LEGO. 1. Substrate-directed synthesis via stereoregular Diels–Alder oligomerizations. J. Am. Chem. Soc. 114, 6330–6353 (1992).
Raymo, F., Kohnke, F. H. & Cardullo, F. The regioselective generation of arynes from polyhalogenobenzenes. An improved synthesis of syn- and anti-1,4,5,8,9,12-hexahydro-1,4:5,8:9,12-triepoxytriphenylene. Tetrahedron 48, 6827–6838 (1992).
Ashton, P. R., Mathias, J. P. & Stoddart, J. F. The oligoselective syntheses of polyacene derivatives. Synthesis 1993, 221–224 (1993).
Ashton, P. R. et al. Molecular belts. 2. Substrate-directed syntheses of belt-type and cage-type structures. J. Am. Chem. Soc. 115, 5422–5429 (1993).
Kohnke, F. H. & Stoddart, J. F. The evolution of molecular belts and collars. Pure Appl. Chem. 61, 1581–1586 (1989).
Kohnke, F. H., Mathias, J. P. & Stoddart, J. F. Structure-directed synthesis of new organic materials. Angew. Chem. Int. Ed. Engl. 28, 1103–1110 (1989).
Ellwood, P., Mathias, J. P., Stoddart, J. F. & Kohnke, F. H. Stereoelectronically-programmed molecular ‘LEGO’ sets. Bull. Soc. Chem. Belg. 97, 669–678 (1988).
Antonsson, T. & Vogel, P. Regioselectivity of 7-oxabicyclo[2.2.1]hepta-2,5-diene-phenol rearrangement as a function of the acid promoter. Stereoselective synthesis of 1,2,3,4-tetrahydro-2-hydroxynaphthalen-2-yl methyl ketones. Tetrahedron Lett. 31, 89–92 (1990).
Tornare, J. M. & Vogel, P. The synthesis of (±)‐11‐deoxydaunomycinone via regioselective tandem Diels–Alder reactions. Helv. Chim. Acta 68, 1069–1077 (1985).
Pinkerton, A. A., Schwarzenbach, D., Stibbard, J. H., Carrupt, P. A. & Vogel, P. Nonplanarity of π systems in 5,6-bis(methylene)-7-oxanorborn-2-ene. J. Am. Chem. Soc. 103, 2095–2096 (1981).
Tornare, J. M., Vogel, P., Pinkerton, A. A. & Schwarzenbach, D. Face selectivity of the Diels–Alder additions of sulfur-substituted dienes and tetraenes grafted onto 7-oxabicyclo[2.2.1]heptanes. Helv. Chim. Acta 68, 2195–2215 (1985).
Brown, F. K. & Houk, K. N. Torsional and steric control of stereoselectivity in isodicyclopentadiene cycloadditions. J. Am. Chem. Soc. 107, 1971–1978 (1985).
Harada, T., Takeuchi, M., Hatsuda, M., Ueda, S. & Oku, A. Effects of torsional angles of 2,2′-biaryldiol ligands in asymmetric Diels–Alder reactions of acrylates catalyzed by their titanium complexes. Tetrahedron Asymmetry 7, 2479–2482 (1996).
Iafe, R. G. & Houk, K. N. Stereoselectivity control by torsional steering in an intramolecular Diels–Alder reaction of vinyl oxocarbenium ions. Org. Lett. 8, 3469–3472 (2006).
Alston, P. V., Ottenbrite, R. M. & Cohen, T. Secondary orbital interactions determining regioselectivity in the Diels–Alder reaction. 3. Disubstituted dienes. J. Org. Chem. 43, 1864–1867 (1978).
Wannere, C. S. et al. The existence of secondary orbital interactions. J. Comput. Chem. 28, 344–361 (2007).
Levandowski, B. J., Svatunek, D., Sohr, B., Mikula, H. & Houk, K. N. Secondary orbital interactions enhance the reactivity of alkynes in Diels–Alder cycloadditions. J. Am. Chem. Soc. 141, 2224–2227 (2019).
Stoddart, J. F. Molecular LEGO. Chem. Br. 24, 1203–1208 (1988).
Stoddart, J. F. The making of molecular belts and collars. J. Inclusion Phenom. Mol. Recognit. Chem. 7, 227–245 (1989).
Kohnke, F. H., Mathias, J. P. & Stoddart, J. F. Structure-directed synthesis of unnatural products. In Proceedings of the International Symposium on Chemical and Biochemical Problems in Molecular Recognition, Exeter (ed. Roberts, S. M.) 241–269 (Royal Society of Chemistry, 1989).
Mathias, J. P. & Stoddart, J. F. Constructing a molecular LEGO set. Chem. Soc. Rev. 21, 215–225 (1992).
Girreser, U. et al. The structure-directed synthesis of cyclacene and polyacene derivatives. Pure Appl. Chem. 65, 119–125 (1993).
Kohnke, F. H., Mathias, J. P. & Stoddart, J. F. Substrate-directed synthesis: the rapid assembly of novel macropolycyclic structures via ttereoregular Diels–Alder oligomerization. In Top. Curr. Chem. (ed. Weber, E.) 1–69 (Springer Berlin Heidelberg, 1993).
Chen, H., Gui, S., Zhang, Y., Liu, Z. & Miao, Q. Synthesis of a hydrogenated zigzag carbon nanobelt. CCS Chem 2, 613–619 (2020).
Binnig, G., Rohrer, H., Gerber, C. & Weibel, E. Tunneling through a controllable vacuum gap. Appl. Phys. Lett. 40, 178–180 (1982).
Binnig, G. & Rohrer, H. Scanning tunneling microscopy—from birth to adolescence (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 26, 606–614 (1987).
Ruska, E. The development of the electron microscope and of electron microscopy (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 26, 595–706 (1987).
Repp, J., Meyer, G., Stojkovic, S. M., Gourdon, A. & Joachim, C. Molecules on insulating films: scanning-tunneling microscopy imaging of individual molecular orbitals. Phys. Rev. Lett. 94, 026803 (2005).
Repp, J., Meyer, G., Paavilainen, S., Olsson, F. E. & Persson, M. Imaging bond formation between a gold atom and pentacene on an insulating surface. Science 312, 1196–1199 (2006).
Binnig, G., Quate, C. F. & Gerber, C. Atomic force microscope. Phys. Rev. Lett. 56, 930–933 (1986).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Eigler, D. M. & Schweizer, E. K. Positioning single atoms with a scanning tunneling microscope. Nature 344, 524–526 (1990).
Okawa, Y. & Aono, M. Nanoscale control of chain polymerization. Nature 409, 683–684 (2001).
Frommer, J. Scanning tunneling microscopy and atomic force microscopy in organic chemistry. Angew. Chem. Int. Ed. Engl. 31, 1298–1328 (1992).
Pavlicek, N. & Gross, L. Generation, manipulation and characterization of molecules by atomic force microscopy. Nat. Rev. Chem. 1, 0005 (2017).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2, 687–691 (2007).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
Moreno, C. et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 360, 199–203 (2018).
Kaiser, K. et al. An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. Science 365, 1299–1301 (2019).
Pavlicek, N. et al. On-surface generation and imaging of arynes by atomic force microscopy. Nat. Chem. 7, 623–628 (2015).
Kruger, J. et al. Decacene: on-surface generation. Angew. Chem. Int. Ed. 56, 11945–11948 (2017).
Gross, L. et al. Atomic force microscopy for molecular structure elucidation. Angew. Chem. Int. Ed. 57, 3888–3908 (2018).
Timmerman, P., Verboom, W. & Reinhoudt, D. N. Resorcinarenes. Tetrahedron 52, 2663–2704 (1996).
Purse, B. W., Shivanyuk, A. & Rebek, J. Resorcin[6]arene as a building block for tubular crystalline state architectures. Chem. Commun. 2002, 2612–2613 (2002).
Zhang, Y., Tong, S. & Wang, M. X. Synthesis and structure of functionalized zigzag hydrocarbon belts. Angew. Chem. Int. Ed. 59, 18151–18155 (2020).
Nishigaki, S. et al. Synthesis of belt- and Möbius-shaped cycloparaphenylenes by rhodium-catalyzed alkyne cyclotrimerization. J. Am. Chem. Soc. 141, 14955–14960 (2019).
Nogami, J. et al. Enantioselective synthesis of planar chiral zigzag-type cyclophenylene belts by rhodium-catalyzed alkyne cyclotrimerization. J. Am. Chem. Soc. 142, 9834–9842 (2020).
Xie, J. et al. Heteroatom-bridged molecular belts as containers. Nat. Commun. 11, 3348 (2020).
Wang, J. & Miao, Q. A tetraazapentacene-pyrene belt: toward synthesis of N-doped zigzag carbon nanobelts. Org. Lett. 21, 10120–10124 (2019).
Tan, M. L. et al. Oxygen and nitrogen-embedded zigzag hydrocarbon belts. Angew. Chem. Int. Ed. 59, 23649–23658 (2020).
Kassaee, M. Z., Rad, A. H. & Amiri, S. S. Carbon–nitrogen nanorings and nanoribbons: a theoretical approach for altering the ground states of cyclacenes and polyacenes. Monatsh. Chem. 141, 1313–1319 (2010).
Winkler, M. & Houk, K. N. Nitrogen-rich oligoacenes: candidates for n-channel organic semiconductors. J. Am. Chem. Soc. 129, 1805–1815 (2007).
Tonzola, C. J., Alam, M. M., Kaminsky, W. & Jenekhe, S. A. New n-type organic semiconductors: synthesis, single crystal structures, cyclic voltammetry, photophysics, electron transport, and electroluminescence of a series of diphenylanthrazolines. J. Am. Chem. Soc. 125, 13548–13558 (2003).
Wu, Y.-T. & Siegel, J. S. Synthesis, structures, and physical properties of aromatic molecular-bowl hydrocarbons. In Polyarenes I (eds Siegel, J. S. & Wu, Y.-T.) 63–120 (Springer Berlin Heidelberg, 2014).
Qiu, Y., Chen, H., Feng, Y., Schott, M. E. & Stoddart, J. F. Stitching up the belt[n]arenes. Chem 6, 826–829 (2020).
Eisenhut, F. et al. Dodecacene generated on surface: reopening of the energy gap. ACS Nano 14, 1011–1017 (2020).
Grommet, A. B., Feller, M. & Klajn, R. Chemical reactivity under nanoconfinement. Nat. Nanotechnol. 15, 256–271 (2020).
Haberhauer, G. & Hoffmann, R. Aromaticity and Other Conjugation Effects (Wiley, 2012).
Angus, J. R. O. & Johnson, R. P. Columnar homoconjugation. J. Org. Chem. 53, 314–317 (1988).
Colwell, C. E., Price, T. W., Stauch, T. & Jasti, R. Strain visualization for strained macrocycles. Chem. Sci. 11, 3923–3930 (2020).
Alder, R. W. & Sessions, R. B. Force field calculations on molecular belts built from cyclohexa-1,4-diene rings. J. Chem. Soc. Perkin Trans. 2, 1849–1854 (1985).
Peeks, M. D., Claridge, T. D. & Anderson, H. L. Aromatic and antiaromatic ring currents in a molecular nanoring. Nature 541, 200–203 (2017).
Rickhaus, M. et al. Global aromaticity at the nanoscale. Nat. Chem. 12, 236–241 (2020).
Türker, L. The effect of cyclization on acenes. J. Mol. Struct. THEOCHEM 531, 333–337 (2000).
Türker, L. The possibility of superaromatic cyclacenes. Polycyclic Aromat. Compd. 4, 231–236 (1995).
Türker, L. An approximate Hückel total π-electron energy formula for benzenoid aromatics. Polycyclic Aromat. Compd. 4, 107–114 (2006).
André, J.-M., Champagne, B., Perpète, E. A. & Guillaume, M. Linear, cyclic, and Möbius strip polyacenes: the influence of the topology on the size-dependent HOMO–LUMO energy gap. Int. J. Quantum Chem. 84, 607–616 (2001).
Türker, L. AM1 treatment of Hückel type cyclacenes. J. Mol. Struct. 407, 217–220 (1997).
Guillaume, M., Champagne, B., Perpète, E. A. & André, J.-M. Möbius strip versus linear and cyclic polyacenes: a Hückel and semiempirical investigation. Theor. Chem. Acc. 105, 431–436 (2001).
Türker, L. Zigzag cyclopolyacenes: a theoretical study. J. Mol. Struct. THEOCHEM 491, 275–280 (1999).
Gutman, I., Biedermann, P. U., IvanovPetrović, V. & Agranat, I. Cyclic conjugation effects in cyclacenes. Polycyclic Aromat. Compd. 8, 189–202 (1996).
Türker, L. Unusual alternation of HMO bond orders in cyclacenes. Polycyclic Aromat. Compd. 8, 67–71 (1996).
Türker, L. MNDO treatment of the Hückel and Möbius types of cyclacenes. J. Mol. Struct. THEOCHEM 454, 83–86 (1998).
Hückel, E. Quantentheoretische beiträge zum benzolproblem. Eur. Phys. J. A 70, 204–286 (1931).
Baird, N. C. Quantum organic photochemistry. II. Resonance and aromaticity in the lowest 3ππ* state of cyclic hydrocarbons. J. Am. Chem. Soc. 94, 4941–4948 (1972).
Heilbronner, E. Hückel molecular orbitals of Möbius-type conformations of annulenes. Tetrahedron Lett. 5, 1923–1928 (1964).
Ajami, D., Oeckler, O., Simon, A. & Herges, R. Synthesis of a Möbius aromatic hydrocarbon. Nature 426, 819–821 (2003).
Liu, C., Ni, Y., Lu, X., Li, G. & Wu, J. Global aromaticity in macrocyclic polyradicaloids: Hückel’s rule or Baird’s rule? Acc. Chem. Res. 52, 2309–2321 (2019).
Chen, Z. & King, R. B. Spherical aromaticity: Recent work on fullerenes, polyhedral boranes, and related structures. Chem. Rev. 105, 3613–3642 (2005).
Kivelson, S. & Chapman, O. L. Polyacene and a new class of quasi-one-dimensional conductors. Phys. Rev. B 28, 7236–7243 (1983).
Freixas, V. M., Oldani, N., Franklin-Mergarejo, R., Tretiak, S. & Fernandez-Alberti, S. Electronic energy relaxation in a photoexcited fully fused edge sharing carbon nanobelt. J. Phys. Chem. Lett. 11, 4711–4719 (2020).
Choi, H. S., Suh, S. B., Cho, S. J. & Kim, K. S. Ionophores and receptors using cation-π interactions: collarenes. Proc. Natl Acad. Sci. USA 95, 12094–12099 (1998).
Kyba, E. P. et al. Host–guest complexation. 1. Concept and illustration. J. Am. Chem. Soc. 99, 2564–2571 (1977).
Cram, D. J. & Cram, J. M. Host-guest chemistry: complexes between organic compounds simulate the substrate selectivity of enzymes. Science 183, 803–809 (1974).
Cram, D. J. The design of molecular hosts, guests, and their complexes (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 27, 1009–1020 (1988).
Lehn, J. Cryptates: inclusion complexes of macropolycyclic receptor molecules. Pure Appl. Chem. 50, 871–892 (1979).
Lehn, J.-M. Design of Organic Complexing Agents Strategies Towards Properties (Springer, 1973).
Lehn, J.-M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 27, 89–112 (1988).
Haver, R. & Anderson, H. L. Synthesis and properties of porphyrin nanotubes. Helv. Chim. Acta 102, e1800211 (2019).
Bols, P. S. & Anderson, H. L. Template-directed synthesis of molecular nanorings and cages. Acc. Chem. Res. 51, 2083–2092 (2018).
Sun, Z. et al. Finite phenine nanotubes with periodic vacancy defects. Science 363, 151–155 (2019).
Bunz, U. H., Menning, S. & Martin, N. para-Connected cyclophenylenes and hemispherical polyarenes: building blocks for single-walled carbon nanotubes? Angew. Chem. Int. Ed. 51, 7094–7101 (2012).
Yu, X. et al. Cap formation engineering: from opened C60 to single-walled carbon nanotubes. Nano Lett. 10, 3343–3349 (2010).
Liu, B. et al. Nearly exclusive growth of small diameter semiconducting single-wall carbon nanotubes from organic chemistry synthetic end-cap molecules. Nano Lett. 15, 586–595 (2015).
Omachi, H., Nakayama, T., Takahashi, E., Segawa, Y. & Itami, K. Initiation of carbon nanotube growth by well-defined carbon nanorings. Nat. Chem. 5, 572–576 (2013).
Sanchez-Valencia, J. R. et al. Controlled synthesis of single-chirality carbon nanotubes. Nature 512, 61–64 (2014).
Scott, L. T. et al. A rational chemical synthesis of C60. Science 295, 1500–1503 (2002).
Koga, Y., Kaneda, T., Saito, Y., Murakami, K. & Itami, K. Synthesis of partially and fully fused polyaromatics by annulative chlorophenylene dimerization. Science 359, 435–439 (2018).
Fort, E. H., Donovan, P. M. & Scott, L. T. Diels–Alder reactivity of polycyclic aromatic hydrocarbon bay regions: implications for metal-free growth of single-chirality carbon nanotubes. J. Am. Chem. Soc. 131, 16006–16007 (2009).
Butterfield, A. M., Gilomen, B. & Siegel, J. S. Kilogram-scale production of corannulene. Org. Process Res. Dev. 16, 664–676 (2012).
Scott, L. T. Exotic chemistry and rational organic syntheses at 1000 °C. J. Org. Chem. 81, 11535–11547 (2016).
Golder, M. R., Zakharov, L. N. & Jasti, R. Stereochemical implications toward the total synthesis of aromatic belts. Pure Appl. Chem. 89, 1603–1617 (2017).
Zhu, C., Kalin, A. J. & Fang, L. Covalent and noncovalent approaches to rigid coplanar π-conjugated molecules and macromolecules. Acc. Chem. Res. 52, 1089–1100 (2019).
Lee, J., Kalin, A. J., Yuan, T., Al-Hashimi, M. & Fang, L. Fully conjugated ladder polymers. Chem. Sci. 8, 2503–2521 (2017).
Teo, Y. C., Lai, H. W. H. & Xia, Y. Synthesis of ladder polymers: developments, challenges, and opportunities. Chem. Eur. J. 23, 14101–14112 (2017).
Scherf, U. & Müllen, K. The synthesis of ladder polymers. Adv. Polym. Sci. 123, 1–40 (1995).
Scherf, U. Ladder-type materials. J. Mater. Chem. 9, 1853–1864 (1999).
Grimsdale, A. C. & Müllen, K. Oligomers and polymers based on bridged phenylenes as electronic materials. Macromol. Rapid Commun. 28, 1676–1702 (2007).
Blatter, K. & Schlüeter, A. D. Ribbon-shaped structures via repetitive Diels–Alder reaction. A polycatafusene. Macromolecules 22, 3506–3508 (1989).
Schlüter, A. D. Ladder polymers: the new generation. Adv. Mater. 3, 282–291 (1991).
Loffler, M., Enkelmann, V. & Schlüter, A. D. A Diels–Alder ladder polymer bridged by imino and ether groups. Acta Polymer 44, 50–53 (1993).
Schlüter, A. D., Loffler, M. & Enkelmann, V. Synthesis of a fully unsaturated all-carbon ladder polymer. Nature 368, 831–834 (1994).
Schlicke, B., Schirmer, H. & Schlüter, A. D. Unsaturated ladder polymers: structural variations and improved molecular weights. Adv. Mater. 7, 544–546 (1995).
Scherf, U. & Müllen, K. Design and synthesis of extended π-systems: monomers, oligomers, polymers. Synthesis 1992, 23–38 (1992).
Wegener, S. & Müllen, K. New ladder polymers via repetitive Diels–Alder reaction under high-pressure. Macromolecules 26, 3037–3040 (1993).
Pollmann, M. & Müllen, K. Semiflexible ribbon-type structures via repetitive Diels–Alder cycloaddition. Cage formation versus polymerization. J. Am. Chem. Soc. 116, 2318–2323 (1994).
Horn, T., Wegener, S. & Müllen, K. Poly[n]acene precursors via repetitive Diels–Alder reactions with dehydrobenzenes. Macromol. Chem. Phys. 196, 2463–2474 (1995).
Thomas, S. W. I. et al. Perpendicular organization of macromolecules: synthesis and alignment studies of a soluble poly(iptycene). J. Am. Chem. Soc. 127, 17976–17977 (2005).
Chen, Z. H., Amara, J. P., Thomas, S. W. I. & Swager, T. M. Synthesis of a novel poly(iptycene) ladder polymer. Macromolecules 39, 3202–3209 (2006).
Perepichka, D. F., Bendikov, M., Meng, H. & Wudl, F. A one-step synthesis of a poly(iptycene) through an unusual Diels–Alder cyclization/dechlorination of tetrachloropentacene. J. Am. Chem. Soc. 125, 10190–10191 (2003).
Narita, A. et al. Synthesis of structurally well-defined and liquid-phase-processable graphene nanoribbons. Nat. Chem. 6, 126–132 (2014).
Ruffieux, P. et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 531, 489–492 (2016).
Talirz, L. et al. On-surface synthesis and characterization of 9-atom wide armchair graphene nanoribbons. ACS Nano 11, 1380–1388 (2017).
Sakaguchi, H., Song, S., Kojima, T. & Nakae, T. Homochiral polymerization-driven selective growth of graphene nanoribbons. Nat. Chem. 9, 57–63 (2017).
Jordan, R. S. et al. Synthesis of N = 8 armchair graphene nanoribbons from four distinct polydiacetylenes. J. Am. Chem. Soc. 139, 15878–15890 (2017).
Matsui, K., Segawa, Y., Namikawa, T., Kamada, K. & Itami, K. Synthesis and properties of all-benzene carbon nanocages: a junction unit of branched carbon nanotubes. Chem. Sci. 4, 84–88 (2013).
Bruns, C. J. & Stoddart, J. F. The Nature of the Mechanical Bond: From Molecules to Machines (Wiley, 2016).
Segawa, Y. et al. Topological molecular nanocarbons: all-benzene catenane and trefoil knot. Science 365, 272–276 (2019).
Fan, Y. Y. et al. An isolable catenane consisting of two Möbius conjugated nanohoops. Nat. Commun. 9, 3037 (2018).
Van Raden, J. M., Jarenwattananon, N. N., Zakharov, L. N. & Jasti, R. Active metal template synthesis and characterization of a nanohoop [c2]daisy chain rotaxane. Chem. Eur. J. 26, 10205–10209 (2020).
Acknowledgements
This Review is dedicated to the memory of François Diederich. We thank K. Itami for giving us advanced access to an article describing the ‘Synthesis of a zigzag carbon nanobelt’. We also thank C. Chi and Y. Han for their helpful comments on our section describing ‘The synthesis of an octabenzo[12]cyclacene’. Q.-H.G., Y.Q. and J.F.S. thank Northwestern University for financial support. M.-X.W. thanks the National Natural Science Foundation of China (22050005) for financial support.
Author information
Authors and Affiliations
Contributions
Q.-H.G. came up with the proposal to write the Review and wrote the first draft. M.-X.W. and J.F.S. oversaw the preparation of the manuscript. Y.Q. contributed the writing of the outlook. All authors contributed to discussions and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, QH., Qiu, Y., Wang, MX. et al. Aromatic hydrocarbon belts. Nat. Chem. 13, 402–419 (2021). https://doi.org/10.1038/s41557-021-00671-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00671-9
This article is cited by
-
Electronic and structural properties of Möbius boron-nitride and carbon nanobelts
Discover Nano (2024)
-
Anomalous anti-Kasha excited-state luminescence from symmetry-breaking heterogeneous carbon bisnanohoops
Nature Communications (2024)
-
Synthesis of covalent organic pillars as molecular nanotubes with precise length, diameter and chirality
Nature Synthesis (2023)
-
Tetrafluorenofulvalene as a sterically frustrated open-shell alkene
Nature Chemistry (2023)
-
Synthesis of an all-carbon conjugated polymeric segment of carbon nanotubes and its application for lithium-ion batteries
Nano Research (2023)