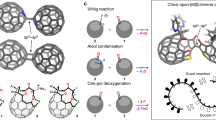
Abstract
Molecular Russian dolls (matryoshkas) have proven useful for testing the limits of preparative supramolecular chemistry but applications of these architectures to problems in other fields are elusive. Here we report a three-shell, matryoshka-like complex—in which C60 sits inside a cycloparaphenylene nanohoop, which in turn is encapsulated inside a self-assembled nanocapsule—that can be used to address a long-standing challenge in fullerene chemistry, namely the selective formation of a particular fullerene bis-adduct. Spectroscopic evidence indicates that the ternary complex is sufficiently stable in solution for the two outer shells to affect the addition chemistry of the fullerene guest. When the complex is subjected to Bingel cyclopropanation conditions, the exclusive formation of a single trans-3 fullerene bis-adduct was observed in a reaction that typically yields more than a dozen products. The selectivity facilitated by this matryoshka-like approach appears to be a general phenomenon and could be useful for applications where regioisomerically pure C60 bis-adducts have been shown to have superior properties compared with isomer mixtures.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its supplementary information files). Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1984575 (C60⊂[10]CPP⊂7·(BArF)8), 1984576 (trans-3-(1-C60)⊂[10]CPP⊂6·(BArF)8) and 1984937 ([Cu2(Me2pTp)(OTf)2](OTf)2). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
He, Y. & Li, Y. Fullerene derivative acceptors for high performance polymer solar cells. Phys. Chem. Chem. Phys. 13, 1970–1983 (2011).
Mishra, A. & Bäuerle, P. Small molecule organic semiconductors on the move: promises for future solar energy technology. Angew. Chem. Int. Ed. 51, 2020–2067 (2012).
Mazzio, K. A. & Luscombe, C. K. The future of organic photovoltaics. Chem. Soc. Rev. 44, 78–90 (2015).
Ragoussi, M.-E. & Torres, T. New generation solar cells: concepts, trends and perspectives. Chem. Commun. 51, 3957–3972 (2015).
Inganäs, O. Organic photovoltaics over three decades. Adv. Mater. 30, 1800388 (2018).
Deng, L.-L., Xie, S.-Y. & Gao, F. Fullerene-based materials for photovoltaic applications: toward efficient, hysteresis-free, and stable perovskite solar cells. Adv. Electron. Mater. 4, 1700435 (2018).
Muñoz, A. et al. Synthesis of giant globular multivalent glycofullerenes as potent inhibitors in a model of Ebola virus infection. Nat. Chem. 8, 50–57 (2015).
Nierengarten, J.-F. et al. Giant glycosidase inhibitors: first- and second-generation fullerodendrimers with a dense iminosugar shell. Chem. Eur. J. 24, 2483–2492 (2018).
Hirsch, A. & Brettreich, M. Fullerenes, Chemistry and Reactions (Wiley-VCH, 2005).
Fuertes-Espinosa, C., Pujals, M. & Ribas, X. Supramolecular purification and regioselective functionalization of fullerenes and endohedral metallofullerenes. Chem 6, 3219–3262 (2020).
Djojo, F., Herzog, A., Lamparth, I., Hampel, F. & Hirsch, A. Regiochemistry of twofold additions to [6,6] bonds in C60: influence of the addend-independent cage distortion in 1,2-monoadducts. Chem. Eur. J. 2, 1537–1547 (1996).
Hirsch, A., Lamparth, I. & Karfunkel, H. R. Fullerene chemistry in three dimensions: isolation of seven regioisomeric bisadducts and chiral trisadducts of C60 and di(ethoxycarbonyl)methylene. Angew. Chem. Int. Ed. 33, 437–438 (1994).
Lenes, M. et al. Fullerene bisadducts for enhanced open-circuit voltages and efficiencies in polymer solar cells. Adv. Mater. 20, 2116–2119 (2008).
Shi, W. et al. Purification and electronic characterisation of 18 isomers of the OPV acceptor material bis-[60]PCBM. Chem. Commun. 53, 975–978 (2017).
Cao, T. et al. Towards a full understanding of regioisomer effects of indene-C60 bisadduct acceptors in bulk heterojunction polymer solar cells. J. Mater. Chem. A 5, 10206–10219 (2017).
Zhang, F. et al. Isomer-pure bis-PCBM-assisted crystal engineering of perovskite solar cells showing excellent efficiency and stability. Adv. Mater. 29, 1606806 (2017).
Umeyama, T. & Imahori, H. Isomer effects of fullerene derivatives on organic photovoltaics and perovskite solar cells. Acc. Chem. Res. 52, 2046–2055 (2019).
Isaacs, L., Diederich, F. & Haldimann, R. F. Multiple adducts of C60 by tether-directed remote functionalization and synthesis of soluble derivatives of new carbon allotropes Cn(60+5). Helv. Chim. Acta 80, 317–342 (1997).
Isaacs, L., Haldimann, R. F. & Diederich, F. Tether-directed remote functionalization of buckminsterfullerene: regiospecific hexaadduct formation. Angew. Chem. Int. Ed. 33, 2339–2342 (1994).
Ðorđević, L. et al. Light-controlled regioselective synthesis of fullerene bis-adducts. Angew. Chem. Int. Ed. 60, 313–320 (2021).
Qian, W. & Rubin, Y. Complete control over addend permutation at all six pseudooctahedral positions of fullerene C60. J. Am. Chem. Soc. 122, 9564–9565 (2000).
Beuerle, F. & Hirsch, A. Synthesis and orthogonal functionalization of [60]fullerene e,e,e-trisadducts with two spherically defined addend zones. Chem. Eur. J. 15, 7434–7446 (2009).
Beuerle, F., Chronakis, N. & Hirsch, A. Regioselective synthesis and zone selective deprotection of [60]fullerene tris-adducts with an e,e,e addition pattern. Chem. Commun. 3676–3678 (2005).
Kräutler, B. et al. A topochemically controlled, regiospecific fullerene bisfunctionalization. Angew. Chem. Int. Ed. 35, 1204–1206 (1996).
Schwenninger, R., Müller, T. & Kräutler, B. Concise route to symmetric multiadducts of [60]fullerene: preparation of an equatorial tetraadduct by orthogonal transposition. J. Am. Chem. Soc. 119, 9317–9318 (1997).
Ortiz, A. L. & Echegoyen, L. Unexpected and selective formation of an (e,e,e,e)-tetrakis-[60]fullerene derivative via electrolytic retro-cyclopropanation of a D2h-hexakis-[60]fullerene adduct. J. Mater. Chem. 21, 1362–1364 (2011).
Hörmann, F., Donaubauer, W., Hampel, F. & Hirsch, A. Efficient synthesis of C2v-symmetrical pentakisadducts of C60 as versatile building blocks for fullerene architectures that involve a mixed octahedral addition pattern. Chem. Eur. J. 18, 3329–3337 (2012).
Yoshizawa, M., Klosterman, J. K. & Fujita, M. Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts. Angew. Chem. Int. Ed. 48, 3418–3438 (2009).
Brenner, W., Ronson, T. K. & Nitschke, J. R. Separation and selective formation of fullerene adducts within an MII8L6 cage. J. Am. Chem. Soc. 139, 75–78 (2017).
Huang, N. et al. Tailor-made pyrazolide-based metal–organic frameworks for selective catalysis. J. Am. Chem. Soc. 140, 6383–6390 (2018).
Chen, B., Holstein, J. J., Horiuchi, S., Hiller, W. G. & Clever, G. H. Pd(ii) coordination sphere engineering: pyridine cages, quinoline bowls, and heteroleptic pills binding one or two fullerenes. J. Am. Chem. Soc. 141, 8907–8913 (2019).
Leonhardt, V., Fimmel, S., Krause, A.-M. & Beuerle, F. A covalent organic cage compound acting as a supramolecular shadow mask for the regioselective functionalization of C60. Chem. Sci. 11, 8409–8415 (2020).
Bottari, G. et al. Regio-, stereo-, and atropselective synthesis of C60 fullerene bisadducts by supramolecular-directed functionalization. Angew. Chem. Int. Ed. 55, 11020–11025 (2016).
Iwamoto, T., Watanabe, Y., Sadahiro, T., Haino, T. & Yamago, S. Size-selective encapsulation of C60 by [10]cycloparaphenylene: formation of the shortest fullerene-peapod. Angew. Chem. Int. Ed. 50, 8342–8344 (2011).
Xia, J., Bacon, J. W. & Jasti, R. Gram-scale synthesis and crystal structures of [8]- and [10]CPP, and the solid-state structure of C60@[10]CPP. Chem. Sci. 3, 3018–3021 (2012).
Xu, Y. et al. A supramolecular [10]CPP junction enables efficient electron transfer in modular porphyrin–[10]CPP⊃fullerene complexes. Angew. Chem. Int. Ed. 57, 11549–11553 (2018).
Rio, J. et al. Electronic communication between two [10]cycloparaphenylenes and bis(azafullerene) (C59N)2 induced by cooperative complexation. Angew. Chem. Int. Ed. 57, 6930–6934 (2018).
Xu, Y. & von Delius, M. The supramolecular chemistry of strained carbon nanohoops. Angew. Chem. Int. Ed. 59, 559–573 (2020).
Xu, Y. et al. Concave–convex π–π template approach enables the synthesis of [10]cycloparaphenylene–fullerene [2]rotaxanes. J. Am. Chem. Soc. 140, 13413–13420 (2018).
Fuertes-Espinosa, C. et al. Supramolecular fullerene sponges as catalytic masks for regioselective functionalization of C60. Chem 6, 169–186 (2020).
Kawase, T., Tanaka, K., Shiono, N., Seirai, Y. & Oda, M. Onion-type complexation based on carbon nanorings and a buckminsterfullerene. Angew. Chem. Int. Ed. 43, 1722–1724 (2004).
Rousseaux, S. A. L. et al. Self-assembly of Russian doll concentric porphyrin nanorings. J. Am. Chem. Soc. 137, 12713–12718 (2015).
Cai, K. et al. Molecular Russian dolls. Nat. Commun. 9, 5275 (2018).
Zhang, D. et al. Enantiopure [Cs+/Xe⊂cryptophane]⊂FeII4L4 hierarchical superstructures. J. Am. Chem. Soc. 141, 8339–8345 (2019).
García-Simón, C. et al. Sponge-like molecular cage for purification of fullerenes. Nat. Commun. 5, 5557 (2014).
Fuertes-Espinosa, C. et al. Purification of uranium-based endohedral metallofullerenes (EMFs) by selective supramolecular encapsulation and release. Angew. Chem. Int. Ed. 57, 11294–11299 (2018).
Park, K. et al. Synthesis of symmetrical and unsymmetrical diarylalkynes from propiolic acid using palladium-catalyzed decarboxylative coupling. J. Org. Chem. 75, 6244–6251 (2010).
Brynn Hibbert, D. & Thordarson, P. The death of the Job plot, transparency, open science and online tools, uncertainty estimation methods and other developments in supramolecular chemistry data analysis. Chem. Commun. 52, 12792–12805 (2016).
Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 40, 1305–1323 (2011).
Thordarson, P. et al. Allosterically driven multicomponent assembly. Angew. Chem. Int. Ed. 43, 4755–4759 (2004).
Rizzuto, F. J. & Nitschke, J. R. Stereochemical plasticity modulates cooperative binding in a CoII12L6 cuboctahedron. Nat. Chem. 9, 903–908 (2017).
García-Simón, C. et al. Complete dynamic reconstruction of C60, C70, and (C59N)2 encapsulation into an adaptable supramolecular nanocapsule. J. Am. Chem. Soc. 142, 16051–16063 (2020).
Dannhäuser, J. et al. σ-donor and π-acceptor stacking interactions in a trans-2-linked C60–cobalt(ii) tetraphenylporphyrin diad. Angew. Chem. Int. Ed. 45, 3368–3372 (2006).
Matsuno, T., Nakai, Y., Sato, S., Maniwa, Y. & Isobe, H. Ratchet-free solid-state inertial rotation of a guest ball in a tight tubular host. Nat. Commun. 9, 1907 (2018).
Mecozzi, S. & Rebek, J. J. The 55% solution: a formula for molecular recognition in the liquid state. Chem. Eur. J. 4, 1016–1022 (1998).
Acknowledgements
This work was supported by grants from MINECO-Spain (CTQ2016-77989-P and PID2019-104498GB-I00 to X.R., RTI2018-095622-B-100 to D.M. and I.I., and EUR2019-103824 to F.G.), Generalitat de Catalunya (2017SGR264 and a PhD grant to C.F.-E.) and the Severo Ochoa Center of Excellence Program (Catalan Institute of Nanoscience and Nanotechnology, grant SEV-2017-0706). X.R. is also grateful for ICREA-Acadèmia awards. M.v.D. is grateful for financial support from the Deutsche Forschungsgemeinschaft (project number 182849149-SFB953 ‘Synthetic Carbon Allotropes’), the Fonds der Chemischen Industrie (FCI), the University of Ulm and the Deutscher Akademischer Austauschdienst (PhD fellowship to O.B.). E.U. thanks Universitat de Girona for a PhD grant and we thank Serveis Tècnics de Recerca, Universitat de Girona for technical support. We thank A. Lledó for artwork assistance and H. Maid (Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany) for assistance with cryoprobe NMR spectroscopy.
Author information
Authors and Affiliations
Contributions
E.U., C.F.-E. and C.G.-S. performed all self-assembly as well as fullerene functionalization experiments and isolated all products. O.B. performed all spectroscopic host–guest titrations and analysed the results with M.v.D. Y.X. synthesized a batch of [10]CPP and the bromomalonate Bingel reagents. L.G. provided technical assistance on HRMS studies. J.J., I.I., F.G. and D.M. technically assisted, performed and solved the XRD structure of C60⊂[10]CPP⊂7·(BArF)8 and trans-3-(1-C60)⊂[10]CPP⊂7·(BArF)8 at the ALBA synchrotron. M.v.D., C.F. and X.R. conceived the project idea. M.v.D and X.R. wrote the manuscript. X.R. directed the project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks T. Barendt, E. Peris and the other, anonymous reviewer(s), for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Self-assembled tetragonal prismatic nanocapsules.
Self-assembly of the Zn-TCPP and the bimetallic macrocyclic clips [M2(Me2p)]4+, [M2(Me2pp)]4+ and [M2(Me2pTp)]4+, to afford the corresponding tetragonal prismatic nanocapsules with increasing cavity-size, that is, 3·(BArF)8, 4·(BArF)8, 5·(BArF)8, 6·(BArF)8, 7·(BArF)8.
Supplementary information
Supplementary Information
Supplementary Figs. 1–96 and Tables 1–17.
Supplementary Data
CIF file for clip complex [Cu2(Me2pTp)(OTf)2](OTf)2 (CCDC reference 1984937).
Supplementary Data
CIF file for C60⊂[10]CPP⊂7·(BArF)8 (CCDC reference 1984575).
Supplementary Data
CIF file for trans-3-(1-C60)⊂[10]CPP⊂7·(BArF)8 (CCDC reference 1984576).
Supplementary Video
Video summary of the article, containing the video representation of the matryoshka-like crystal structure and the selectivity for the trans-3 C60 bis-adduct.
Rights and permissions
About this article
Cite this article
Ubasart, E., Borodin, O., Fuertes-Espinosa, C. et al. A three-shell supramolecular complex enables the symmetry-mismatched chemo- and regioselective bis-functionalization of C60. Nat. Chem. 13, 420–427 (2021). https://doi.org/10.1038/s41557-021-00658-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-021-00658-6
This article is cited by
-
Enantioselective fullerene functionalization through stereochemical information transfer from a self-assembled cage
Nature Chemistry (2023)
-
Highly emissive tribenzotriquinacene-based double-rimed nanocube
Nano Research (2023)
-
Electrostatically cooperative host-in-host of metal cluster ⊂ ionic organic cages in nanopores for enhanced catalysis
Nature Communications (2022)