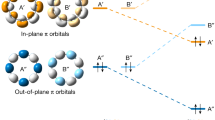
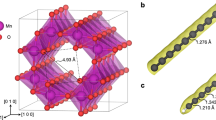
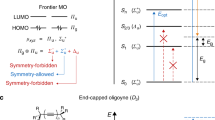
Abstract
The versatility of carbon is revealed in its all-carbon forms (allotropes), which feature unique properties (consider the differences between diamond, graphite, graphene and fullerenes). Beyond natural sources, there are many opportunities to expand the realm of carbon chemistry through the study of new carbon forms. In this work, the synthesis of oligo-/polyynes is used to model the elusive carbyne. The chemical stabilization of oligoynes by sterically encumbered endgroups, particularly the 3,5-bis(3,5-di-tert-butylphenyl)pyridyl group, is key to assemble an extended series of stable oligoynes. The final member of this series is the longest monodisperse polyyne isolated and characterized so far, featuring 24 contiguous alkyne units (48 carbons). Spectroscopic and X-ray crystallographic analysis show that endgroups influence the properties of oligoyne derivatives, but this effect diminishes as length increases toward the polyyne/carbyne limit. For instance, with ultraviolet–visible spectroscopy, molecular symmetry clearly documents the evolution of characteristics from oligoynes to polyynes (in which endgroup effects are absent). The combined experimental data are used to refine predictions for the D∞h structure of carbyne.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this Article (and its Supplementary Information). The structures of tBu[5], tBu[6], Py**[2c], Py**[2e], Py**[2a], Py**[4a], Py**[6a] (P-1), Py**[6a] (P21/c) and Py**[8a]in the solid state were determined by single-crystal X-ray diffraction, and the crystallographic data have been deposited with the Cambridge Crystallographic Data Centre under CCDC numbers 1981170 (tBu[5]), 1981171 (tBu[6]), 1977432 (Py**[2c]), 1977434 (Py**[2e]), 1977437 (Py**[2a]), 1977437 (Py**[4a]), 1977438 (Py**[6a], P-1), 1977436 (Py**[6a], P21/c) and 1977435 (Py**[8a]). Copies of the data can be obtained free of charge on application to CCDC.
References
Hirsch, A. The era of carbon allotropes. Nat. Mater. 9, 868–871 (2010).
Kaiser, K. et al. An sp-hybridized molecular carbon allotrope, cyclo[18]carbon. Science 365, 1299–1301 (2019).
Kilde, M. D. et al. Synthesis of radiaannulene oligomers to model the elusive carbon allotrope 6,6,12-graphyne. Nat. Commun. 10, 3714 (2019).
Malko, D., Neiss, C., Viñes, F. & Görling, A. Competition for graphene: graphynes with direction-dependent Dirac cones. Phys. Rev. Lett. 108, 086804 (2012).
Wang, X.-Y., Yao, X. & Müllen, K. Polycyclic aromatic hydrocarbons in the graphene era. Sci. China Chem. 62, 1099–1144 (2019).
Schwertfeger, H., Fokin, A. A. & Schreiner, P. R. Diamonds are a chemist’s best friend: diamondoid chemistry beyond adamantane. Angew. Chem. Int. Ed. 47, 1022–1036 (2008).
Darzi, E. R. & Jasti, R. The dynamic, size-dependent properties of [5]–[12]cycloparaphenylenes. Chem. Soc. Rev. 44, 6401–6410 (2015).
Chalifoux, W. A. & Tykwinski, R. R. Synthesis of extended polyynes: toward carbyne. C.R. Chim. 12, 341–358 (2009).
Tykwinski, R. R. Carbyne: the molecular approach. Chem. Rec. 15, 1060–1074 (2015).
Szafert, S. & Gladysz, J. A. Update 1 of: carbon in one dimension: structural analysis of the higher conjugated polyynes. Chem. Rev. 106, PR1–PR33 (2006).
Casari, C. S., Tommasini, M., Tykwinski, R. R. & Milani, A. Carbon-atom wires: 1-D systems with tunable properties. Nanoscale 8, 4414–4435 (2016).
Banhart, F. Elemental carbon in the sp1 hybridization. ChemTexts 6, 3 (2020).
Liu, M., Artyukhov, V. I., Lee, H., Xu, F. & Yakobson, B. I. Carbyne from first principles: chain of C atoms, a nanorod or a nanorope. ACS Nano 7, 10075–10082 (2013).
Zheng, Q. et al. A synthetic breakthrough into an unanticipated stability regime: a series of isolable complexes in which C6, C8, C10, C12, C16, C20, C24, and C28 polyynediyl chains span two platinum atoms. Chem. Eur. J. 12, 6486–6505 (2006).
Weisbach, N. et al. Triisopropylsilyl (TIPS) alkynes as building blocks for syntheses of platinum triisopropylsilylpolyynyl and diplatinum polyynediyl complexes. Organometallics 38, 3294–3310 (2019).
Chalifoux, W. A. & Tykwinski, R. R. Synthesis of polyynes to model the sp-carbon allotrope carbyne. Nat. Chem. 2, 967–971 (2010).
Shi, L. et al. Confined linear carbon chains as a route to bulk carbyne. Nat. Mater. 15, 634–639 (2016).
Pigulski, B., Gulia, N. & Szafert, S. Reactivity of polyynes: complex molecules from simple carbon rods. Eur. J. Org. Chem. 2019, 1420–1445 (2019).
Wang, C. S. et al. Oligoyne single molecule wires. J. Am. Chem. Soc. 131, 15647–15654 (2009).
Milan, D. C. et al. The single-molecule electrical conductance of a rotaxane-hexayne supramolecular assembly. Nanoscale 9, 355–361 (2017).
Moreno-García, P. et al. Single-molecule conductance of functionalized oligoynes: length dependence and junction evolution. J. Am. Chem. Soc. 135, 12228–12240 (2013).
Wang, C., Jia, H., Li, H. & Wang, Y. 1,12-bis(4-Pyridyl)-1,3,5,7,9,11-dodecyl-hexayne: synthesis and properties. Jilin Huagong Xueyuan Xuebao 20, 33–36 (2012).
Krempe, M. et al. Pyridyl-endcapped polyynes: stabilized wire-like molecules. Angew. Chem. Int. Ed. 55, 14802–14806 (2016).
Simpkins, S. M. E., Weller, M. D. & Cox, L. R. β-Chlorovinylsilanes as masked alkynes in oligoyne assembly: synthesis of the first aryl-end-capped dodecayne. Chem. Commun. 4035–4037 (2007).
Robke, L. et al. Discovery of 2,4-dimethoxypyridines as novel autophagy inhibitors. Tetrahedron 74, 4531–4537 (2018).
Eglinton, G. & Galbraith, A. R. Cyclic diynes. Chem. Ind. (London) 737–738 (1956).
Eisler, S. et al. Polyynes as a model for carbyne: synthesis, physical properties, and nonlinear optical response. J. Am. Chem. Soc. 127, 2666–2676 (2005).
Gibtner, T., Hampel, F., Gisselbrecht, J. P. & Hirsch, A. End-cap stabilized oligoynes: model compounds for the linear sp carbon allotrope carbyne. Chem. Eur. J. 8, 408–432 (2002).
Movsisyan, L. D. et al. Polyyne rotaxanes: stabilization by encapsulation. J. Am. Chem. Soc. 138, 1366–1376 (2016).
Biradha, K. & Santra, R. Crystal engineering of topochemical solid state reactions. Chem. Soc. Rev. 42, 950–967 (2013).
Chalifoux, W. A., McDonald, R., Ferguson, M. J. & Tykwinski, R. R. tert-Butyl-end-capped polyynes: crystallographic evidence of reduced bond-length alternation. Angew. Chem. Int. Ed. 48, 7915–7919 (2009).
Bredas, J.-L. Mind the gap! Mater. Horiz. 1, 17–19 (2014).
Zirzlmeier, J. et al. Optical gap and fundamental gap of oligoynes and carbyne. Nat. Commun. 11, 4797 (2020).
Milani, A., Tommasini, M. & Zerbi, G. Connection among Raman wavenumbers, bond length alternation and energy gap in polyynes. J. Raman Spectrosc. 40, 1931–1934 (2009).
Kertesz, M., Choi, C. H. & Yang, S. Conjugated polymers and aromaticity. Chem. Rev. 105, 3448–3481 (2005).
Shi, L. et al. Electronic band gaps of confined linear carbon chains ranging from polyyne to carbyne. Phys. Rev. Mater. 1, 075601 (2017).
Hoffmann, R. How chemistry and physics meet in the solid state. Angew. Chem. Int. Ed. 26, 846–878 (1987).
Tykwinski, R. R. et al. Toward carbyne: synthesis and stability of really long polyynes. Pure Appl. Chem. 82, 891–904 (2010).
Movsisyan, L. D. et al. Photophysics of threaded sp-carbon chains: the polyyne is a sink for singlet and triplet excitation. J. Am. Chem. Soc. 136, 17996–18008 (2014).
Fazzi, D. et al. Ultrafast spectroscopy of linear carbon chains: the case of dinaphthylpolyynes. Phys. Chem. Chem. Phys. 15, 9384–9391 (2013).
Nagano, Y., Ikoma, T., Akiyama, K. & Tero-Kubota, S. Symmetry switching of the fluorescent excited state in α,ω-diphenylpolyynes. J. Am. Chem. Soc. 125, 14103–14112 (2003).
Meier, H., Stalmach, U. & Kolshorn, H. Effective conjugation length and UV/vis spectra of oligomers. Acta Polym. 48, 379–384 (1997).
Martin, R. E. & Diederich, F. Linear monodisperse π-conjugated oligomers: model compounds for polymers and more. Angew. Chem. Int. Ed. 38, 1350–1377 (1999).
Lucotti, A. et al. Evidence for solution-state nonlinearity of sp-carbon chains based on IR and Raman spectroscopy: violation of mutual exclusion. J. Am. Chem. Soc. 131, 4239–4244 (2009).
Gieseking, R. L., Risko, C. & Brédas, J.-L. Distinguishing the effects of bond-length alternation versus bond-order alternation on the nonlinear optical properties of π-conjugated chromophores. J. Phys. Chem. Lett. 6, 2158–2162 (2015).
Zeinalipour-Yazdi, C. D. & Pullman, D. P. Quantitative structure-property relationships for longitudinal, transverse, and molecular static polarizabilities in polyynes. J. Phys. Chem. B 112, 7377–7386 (2008).
Yang, S. & Kertesz, M. Bond length alternation and energy band gap of polyyne. J. Phys. Chem. A 110, 9771–9774 (2006).
Agarwal, N. R. et al. Structure and chain polarization of long polyynes investigated with infrared and Raman spectroscopy. J. Raman Spectrosc. 44, 1398–1410 (2013).
Acknowledgements
This Article is dedicated to the memory of François Diederich. We are grateful for funding from the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Foundation for Innovation (CFI), and the Deutsche Forschungsgemeinschaft (SFB 953, Synthetic Carbon Allotropes). We thank S. Frankenburger for synthesis and F. Hampel for X-ray structural determination of tBu[5] and tBu[6], Y. Zhou and R. McDonald for help with acquisition of the X-ray diffraction data for Py**[2a], Py**[6a] and Py**[2e], and L. Chen for crystallization of Py**[6a] (P21/c). Y.H. and Y.G. acknowledge funding from the China Scholarship Council (CSC). J.C. acknowledges MINECO and Junta de Andalucía of Spain project references PGC2018-098533-B-I00 and UMA18FEDERJA057.
Author information
Authors and Affiliations
Contributions
R.R.T. designed and oversaw the project. R.R.T and Y.G. designed the molecules, Y.G. and Y.H. synthesized and characterized the molecules. Y.G. carried out room temperature spectroscopy, thermal analyses and data analysis. J.C. and F.G.G. carried out low-temperature absorption and Raman spectroscopy. M.F. conducted X-ray crystallographic characterization, refinement and analysis. Y.G and R.R.T. wrote the paper with contributions from all authors. All authors analysed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–93 and Supplementary Tables 1–21
Supplementary Data 1
Crystallographic data (CIF) for compound tBu[5]; CCDC reference: 1981170
Supplementary Data 2
Crystallographic data (structure factors, FCF) for compound tBu[5]; CCDC reference: 1981170
Supplementary Data 3
Crystallographic data (CIF) for compound tBu[6]; CCDC reference: 1981171
Supplementary Data 4
Crystallographic data (structure factors, FCF) for compound tBu[6]; CCDC reference: 1981171
Supplementary Data 5
Crystallographic data (CIF) for compound Py**[2a]; CCDC reference: 1977437
Supplementary Data 6
Crystallographic data (CIF) for compound Py**[2c]; CCDC reference: 1977432
Supplementary Data 7
Crystallographic data (CIF) for compound Py**[2e]; CCDC reference: 1977434
Supplementary Data 8
Crystallographic data (CIF) for compound Py**[4a]; CCDC reference: 1977433
Supplementary Data 9
Crystallographic data (CIF) for compound Py**[6a] (P-1); CCDC reference: 1977438
Supplementary Data 10
Crystallographic data (CIF) for compound Py**[6a](P21/c); CCDC reference: 1977436
Supplementary Data 11
Crystallographic data (CIF) for compound Py**[8a]; CCDC reference: 1977435
Rights and permissions
About this article
Cite this article
Gao, Y., Hou, Y., Gordillo Gámez, F. et al. The loss of endgroup effects in long pyridyl-endcapped oligoynes on the way to carbyne. Nat. Chem. 12, 1143–1149 (2020). https://doi.org/10.1038/s41557-020-0550-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0550-0
This article is cited by
-
Masked alkynes for synthesis of threaded carbon chains
Nature Chemistry (2024)
-
Interlocked polyynes towards stable carbynes
Nature Chemistry (2024)
-
A robust synthesis route of confined carbyne
Nano Research (2024)
-
Synthesizing γ-graphyne
Nature Synthesis (2022)
-
Doubly linked chiral phenanthrene oligomers for homogeneously π-extended helicenes with large effective conjugation length
Nature Communications (2022)