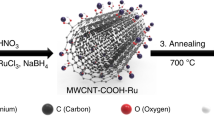
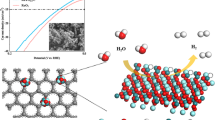
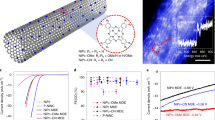
Abstract
Photoelectrochemical cells that utilize water as a source of electrons are one of the most attractive solutions for the replacement of fossil fuels by clean and sustainable solar fuels. To achieve this, heterogeneous water oxidation catalysis needs to be mastered and properly understood. The search continues for a catalyst that is stable at the surface of electro(photo)anodes and can efficiently perform this reaction at the desired neutral pH. Here, we show how oligomeric Ru complexes can be anchored on the surfaces of graphitic materials through CH–π interactions between the auxiliary ligands bonded to Ru and the hexagonal rings of the graphitic surfaces, providing control of their molecular coverage. These hybrid molecular materials behave as molecular electroanodes that catalyse water oxidation to dioxygen at pH 7 with high current densities. This strategy for the anchoring of molecular catalysts on graphitic surfaces can potentially be extended to other transition metals and other catalytic reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated during this study, experimental details together with additional, analytic, spectroscopic, electrochemical, X-ray scattering, microscopy and DFT data are included in this article or in the Supplementary Information. Data for Figs. 1–6 are available as source data with this paper. Data for Supplementary Figs. are available from the corresponding author on reasonable request.
The crystal data parameters of 1 and 2 and all information related to the structures can be found in the deposited CIF/Checkcif-files. CCDC 1945004 (1) and 1945005 (2) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/structures.
A data set collection of computational results is available as source data with this paper and can be accessed at ioChem-BD repository via https://doi.org/10.19061/iochem-bd-1-180. Source data are provided with this paper.
References
Blakemore, J. D., Crabtree, R. H. & Brudvig, G. W. Molecular catalysts for water oxidation. Chem. Rev. 115, 12974–13005 (2015).
Berardi, S. et al. Molecular artificial photosynthesis. Chem. Soc. Rev. 43, 7501–7519 (2014).
Garrido-Barros, P., Gimbert-Suriñach, C., Matheu, R., Sala, X. & Llobet, A. How to make an efficient and robust molecular catalyst for water oxidation. Chem. Soc. Rev. 46, 6088–6098 (2017).
Matheu, R. et al. The development of molecular water oxidation catalysts. Nat. Rev. Chem. 3, 331–341 (2019).
Duan, L. et al. A molecular ruthenium catalyst with water-oxidation activity comparable to that of photosystem II. Nat. Chem. 4, 418–423 (2012).
Suga, M. et al. Native structure of photosystem II at 1.95 Å resolution viewed by femtosecond X-ray pulses. Nature 517, 99–103 (2015).
Matheu, R., Ertem, Z. M., Gimbert-Suriñach, C., Sala, X. & Llobet, A. Seven coordinated molecular ruthenium–water oxidation catalysts: A coordination chemistry journey. Chem. Rev. 119, 3453–3471 (2019).
Garrido-Barros, P. et al. Redox non-innocent ligand controls water oxidation overpotential in a new family of mononuclear Cu-based efficient catalysts. J. Am. Chem. Soc. 137, 6758–6761 (2015).
Grätzel, M. Artificial photosynthesis: water cleavage into hydrogen and oxygen by visible light. Acc. Chem. Res. 14, 376–384 (1981).
Toshima, N. & Yonezawa, T. Bimetallic nanoparticles-novel materials for chemical and physical applications. New J. Chem. 22, 1179–1201 (1998).
Cao, S., Tao, F., Tang, Y., Lia, Y. & Yu, J. Size- and shape-dependent catalytic performances of oxidation and reduction reactions on nanocatalysts. Chem. Soc. Rev. 45, 4747–4765 (2016).
Kotani, H., Hanazaki, R., Ohkubo, K., Yamada, Y. & Fukuzumi, S. Size- and shape-dependent activity of metal nanoparticles as hydrogen evolution catalysts: Mechanistic insights into photocatalytic hydrogen evolution. Chem. Eur. J. 17, 2777–2785 (2011).
Roy, C. et al. Impact of nanoparticle size and lattice oxygen on water oxidation on NiFeOxHy. Nat. Catal. 1, 820–829 (2018).
Francàs, L. et al. Ru–bis(pyridine)pyrazolate (bpp)‐based water‐oxidation catalysts anchored on TiO2: The importance of the nature and position of the anchoring group. Chem. Eur. J. 22, 5261–5268 (2016).
Hyde, J. T. et al. Electrochemical instability of phosphonate-derivatized, ruthenium(iii) polypyridyl complexes on metal oxide surfaces. ACS Appl. Mater. Interfaces 7, 9554–9562 (2015).
Wadsworth, B. L., Beiler, A. M., Khusnutdinova, D., Jacob, S. I. & Moore, G. F. Electrocatalytic and optical properties of cobaloxime catalysts immobilized at a surface-grafted polymer interface. ACS Catal. 6, 8048–8057 (2016).
Chen, Z., Concepcion, J. J., Jurss, J. W. & Meyer, T. J. Single-site, catalytic water oxidation on oxide surfaces. J. Am. Chem. Soc. 131, 15580–15581 (2009).
Ashford, D. L. et al. Molecular chromophore-catalyst assemblies for solar fuel applications. Chem. Rev. 115, 13006–13049 (2015).
Wu, L. et al. A molecular silane-derivatized Ru(ii) catalyst for photoelectrochemical water oxidation. J. Am. Chem. Soc. 140, 15062–15069 (2018).
Kaminsky, C. J., Wright, J. & Surendranath, Y. Graphite-conjugation enhances porphyrin electrocatalysis. ACS Catal. 9, 3667–3671 (2019).
Jackson, M. N. et al. Strong electronic coupling of molecular sites to graphitic electrodes via pyrazine conjugation. J. Am. Chem. Soc. 140, 1004–1010 (2018).
Oh, S., Gallagher, J. R., Miller, J. T. & Surendranath, Y. Graphite-conjugated rhenium catalysts for carbon dioxide reduction. J. Am. Chem. Soc. 138, 1820–1823 (2016).
Blakemore, J. D., Gupta, A., Warren, J. J., Brunschwig, B. S. & Gray, H. B. Noncovalent immobilization of electrocatalysts on carbon electrodes for fuel production. J. Am. Chem. Soc. 135, 18288–18291 (2013).
Sheehan, S. W. et al. A molecular catalyst for water oxidation that binds to metal oxide surfaces. Nat. Commun. 6, 6469 (2015).
Mitchell, S., Thomas, J. M. & Pérez-Ramírez, J. Single atom catalysis. Catal. Sci. Technol. 7, 4248–4249 (2017).
Sala, X. et al. Molecular water oxidation mechanisms followed by transition metals: State of the art. Acc. Chem. Res. 47, 504–516 (2014).
Garrido-Barros, P., Matheu, R., Gimbert-Suriñach, C. & Llobet, A. Electronic, mechanistic and structural factors that influence the performance of molecular water oxidation catalysts anchored on electrode surfaces. Curr. Opin. Electrochem. 15, 140–147 (2019).
Creus, J. et al. A million turnover molecular anode for catalytic water oxidation. Angew. Chem. Int. Ed. 55, 15382–15386 (2016).
McCrory, C. C. L., Jung, S., Peters, J. C. & Jaramillo, T. F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 135, 16977–16987 (2013).
McCrory, C. C. L. et al. Benchmarking hydrogen evolving reaction and oxygen evolving reaction electrocatalysts for solar water splitting devices. J. Am. Chem. Soc. 137, 4347–4357 (2015).
Smith, D. L. et al. Photochemical route for accessing amorphous metal oxide materials for water oxidation catalysis. Science 340, 60–63 (2013).
Merrill, M. D. & Dougherty, R. C. Metal oxide catalysts for the evolution of O2 from H2O. J. Phys. Chem. C 112, 3655–3666 (2008).
Smith, R. D. L., Prevot, M. S., Fagan, R. D., Trudel, S. & Berlinguette, C. P. Water oxidation catalysis: electrocatalytic response to metal stoichiometry in amorphous metal oxide films containing iron, cobalt, and nickel. J. Am. Chem. Soc. 135, 11580–11586 (2013).
Salvatore, D. A., Pena, B., Dettelbach, K. E. & Berlinguette, C. P. Photodeposited ruthenium dioxide films for oxygen evolution reaction electrocatalysis. J. Mater. Chem. A 5, 1575–1580 (2017).
Carmo, M., Fritz, D. L., Mergel, J. & Stolten, D. A comprehensive review on PEM water electrolysis. J. Hydrogen Energy 38, 4901–4934 (2013).
Zeng, K. & Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 36, 307–326 (2010).
Xu, J. et al. Cluster beam deposition of ultrafine cobalt and ruthenium clusters for efficient and stable oxygen evolution reaction. ACS Appl. Energy Mater. 1, 3013–3018 (2018).
Li, W. et al. From water reduction to oxidation: Janus Co–Ni–P nanowires as high-efficiency and ultrastable electrocatalysts for over 3000 h water splitting. J. Power Sources 330, 156–166 (2016).
Kanan, M. W. & Nocera, D. G. In situ formation of an oxygen-evolving catalyst in neutral water containing phosphate and Co2+. Science 321, 1072–1075 (2008).
Surendranath, Y., Kanan, M. W. & Nocera, D. G. Mechanistic studies of the oxygen evolution reaction by a cobalt-phosphate catalyst at neutral pH. J. Am. Chem. Soc. 132, 16501–16509 (2010).
Hoffman, R. E. et al. Self-diffusion measurements of polycyclic aromatic hydrocarbon alkali metal salts. J. Chem. Soc. Perkin Trans. 2, 1659–1664 (1998).
Liu, T. & Xiao, Z. Dynamic light scattering of rigid rods — A universal relationship on the apparent diffusion coefficient as revealed by numerical studies and its use for rod length determination. Macromol. Chem. Phys. 213, 1697–1705 (2012).
Ma, J. et al. Amorphous FeNi-bimetallic infinite coordination polymers as advanced electrocatalysts for the oxygen evolution reaction. Chem. Commun. 55, 12567–12570 (2019).
Bhunia, S. et al. Efficacious electrochemical oxygen evolution from a novel Co(II) porphyrin/pyrene-based conjugated microporous polymer. ACS Appl. Mater. Interfaces 11, 1520–1528 (2019).
Matheu, R. et al. Intramolecular proton transfer boosts water oxidation catalyzed by a Ru complex. J. Am. Chem. Soc. 137, 10786–10795 (2015).
Neel, A. J., Hilton, M. J., Sigman, M. S. & Toste, F. D. Exploiting non-covalent π interactions for catalyst design. Nature 543, 637–646 (2017).
Nishio, M. The CH/– hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 13, 13873–13900 (2011).
Xiao, Y. & Lu, X. Morphology of organic photovoltaic non-fullerene acceptors investigated by grazing incidence X-ray scattering techniques. Mater. Today Nano 5, 100030 (2019).
Rodríguez-Guerra Pedregal, J. et al. GARLEEK: Adding an extra flavor to ONIOM. J. Comput. Chem. 40, 381–386 (2019).
Álvarez-Moreno, M. et al. Managing the computational chemistry big data problem: the ioChem-BD platform. J. Chem. Inf. Model. 55, 95–103 (2015).
Waters, M. L. Aromatic interactions in model systems. Curr. Opin. Chem. Biol. 6, 736–741 (2002).
Concepcion, J. J., Jurss, J. W., Hoertz, P. G. & Meyer, T. J. Catalytic and surface-electrocatalytic water oxidation by redox mediator–catalyst assemblies. Angew. Chem. Int. Ed. 48, 9473–9476 (2009).
Matheu, R., Benet-Buchholz, J., Sala, X. & Llobet, A. Synthesis, structure, and redox properties of a trans-diaqua Ru complex that reaches seven-coordination at high oxidation states. Inorg. Chem. 57, 1757–1765 (2018).
Noviandri, I. et al. The decamethylferrocenium/decamethylferrocene redox couple: A superior redox standard to the ferrocenium/ferrocene redox couple for studying solvent effects on the thermodynamics of electron transfer. J. Phys. Chem. B 103, 6713–6722 (1999).
Simonelli, L. et al. CLÆSS: The hard X-ray absorption beamline of the ALBA CELLS synchrotron. Cogent Phys. 3, 1231987 (2016).
Kieffer, J. & Karkoulis, D. PyFAI, a versatile library for azimuthal regrouping. J. Phys. Conf. Ser. 425, 202012 (2013).
Konarev, P. V., Volkov, V. V., Sokolova, A. V., Koch, M. H. J. & Svergun, D. I. PRIMUS: a Windows-PC based system for small-angle scattering data analysis. J. Appl. Cryst. 36, 1277–1282 (2003).
Hulsken, B. et al. Real-time single-molecule imaging of oxidation catalysis at a liquid–solid interface. Nat. Nanotechnol. 2, 285–289 (2007).
Acknowledgements
Md.A.H. acknowledge funding from AGAUR with grant nos 2016FI_B 01011 and 2014 SGR-915. F.M. and A.d.A. acknowledge funding from MINECO (CTQ2017-87792-R). A.d.A. thanks MINECO for a FPI fellowship (BES-2015-073012). J.L. thanks the Alexander von Humboldt Foundation for financial support. D.M. acknowledges support by the Severo Ochoa Excellence programme (SEV-2016-0686) from the Instituto IMDEA Nanociencia, the Acciones de Dinamización “Europa Investigacion” grant (EIN2019-103399) and the Spanish Ministerio de Ciencia, Innovación y Universidades grant (PID2019-111086RA-I00). X.S. acknowledges funding from MINECO/FEDER (PID2019-104171RB-I00). A.L. acknowledges support from the Ministerio de Ciencia e Innovación, FEDER and AGAUR through grants: PID2019-111617RB-I00 and 2017-SGR-1631. XAS experiments were performed at the CLAESS beamline at the ALBA Synchrotron under proposal No. 2016091818 and 2017092493 with the collaboration of ALBA staff and additionally used resources of the sector 20 beamline at the APS at Argonne National Laboratory. Sector 20 beamline at APS is operated by the US DOE (Contract No. DE-AC02-06CH11357) and the Canadian Light Source. Synchrotron X-ray scattering experiments were performed at NCD-SWEET beamline at the ALBA synchrotron with the collaboration of ALBA staff (Proposal 2020014050).
Author information
Authors and Affiliations
Contributions
Md.A.H. and M.G.-S. performed the synthesis, characterization and electrochemical experiments and coordinated the tasks with all authors. These authors contributed equally to this work. J.A.A.W.E. performed the STM experiments. D.M. performed the XANES and EXAFS measurements and data analysis. Y.S. prepared the samples for XANES and EXAFS experiments. J.B.-B. performed the single crystal X-ray structure determinations. M.M. and E.S. performed and analysed the synchrotron scattering experiments. J.L., A.G.-M. and C.S. designed, carried out and analysed electron microscopy experiments. F.M. designed the computational part. A.d.A. performed the theoretical calculations. C.G.-S. supervised the project. A.L. conceived the idea of the project and wrote the paper with input from other authors. All authors contributed to the design of experiments, analysis of the results and preparation of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary methods, Figs. 1–48, computational methods.
Supplementary Data 1
Supplementary Data 1.
Supplementary Data 2
Supplementary Data 2.
Supplementary Data 3
Supplementary Data 3.
Source data
Source Data Fig. 1
This file contains raw NMR data for Fig. 1.
Source Data Fig. 3
Figure_3D.xlsx – Excel file with source data for Fig. 3d.
Source Data Fig. 4
Coordinates for this figure.
Source Data Fig. 5
Figure_5.xlsx – Excel file with source data for Fig. 5.
Source Data Fig. 6
Excel file with source data for Fig. 6.
Rights and permissions
About this article
Cite this article
Hoque, M.A., Gil-Sepulcre, M., de Aguirre, A. et al. Water oxidation electrocatalysis using ruthenium coordination oligomers adsorbed on multiwalled carbon nanotubes. Nat. Chem. 12, 1060–1066 (2020). https://doi.org/10.1038/s41557-020-0548-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0548-7