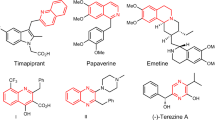
Abstract
Intermolecular [2+2] photocycloadditions represent a powerful method for the synthesis of highly strained, four-membered rings. Although this approach is commonly employed for the synthesis of oxetanes and cyclobutanes, the synthesis of azetidines via intermolecular aza Paternò–Büchi reactions remains highly underdeveloped. Here we report a visible-light-mediated intermolecular aza Paternò–Büchi reaction that utilizes the unique triplet state reactivity of oximes, specifically 2-isoxazoline-3-carboxylates. The reactivity of this class of oximes can be harnessed via the triplet energy transfer from a commercially available iridium photocatalyst and allows for [2+2] cycloaddition with a wide range of alkenes. This approach is characterized by its operational simplicity, mild conditions and broad scope, and allows for the synthesis of highly functionalized azetidines from readily available precursors. Importantly, the accessible azetidine products can be readily converted into free, unprotected azetidines, which represents a new approach to access these highly desirable synthetic targets.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Experimental data as well as characterization data for all new compounds prepared in the course of these studies are provided in the Supplementary Information of this manuscript. The X-ray crystallographic coordinates for compounds 44, 46 and 59 have been deposited at the Cambridge Crystallographic Data Center (CCDC) with accession codes 1980947 (44), 1980951 (46) and 1980952 (59). These data can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/structures/.
References
Fish, P. V., Brown, A. D., Evrard, E. & Roberts, L. R. 7-Sulfonamido-3-benzazepines as potent and selective 5-HT2C receptor agonists: hit-to-lead optimization. Bioorg. Med. Chem. Lett. 19, 1871–1875 (2009).
Brown, A. et al. Triazole oxytocin antagonists: identification of an aryloxyazetidine replacement for a biaryl substituent. Bioorg. Med. Chem. Lett. 20, 516–520 (2010).
Lowe, J. T. et al. Synthesis and profiling of a diverse collection of azetidine-based scaffolds for the development of CNS-focused lead-like libraries. J. Org. Chem. 77, 7187–7211 (2012).
Maetani, M. et al. Synthesis of a bicyclic azetidine with in vivo antimalarial activity enabled by stereospecific, directed C(sp3)–H arylation. J. Am. Chem. Soc. 139, 11300–11306 (2017).
Kerns, E. H. & Di, L. Drug-Like Properties: Concepts, Structure Design and Methods 1st edn 137–168 (Academic, 2008).
St. Jean, D. J. & Fotsch, C. Mitigating heterocycle metabolism in drug discovery. J. Med. Chem. 55, 6002–6020 (2012).
Shu, Y.-Z., Johnson, B. M. & Yang, T. J. Role of biotransformation studies in minimizing metabolism-related liabilities in drug discovery. AAPS J. 10, 178–192 (2008).
Lovering, F., Bikker, J. & Humblet, C. Escape from Flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Lovering, F. Escape from Flatland 2: complexity and promiscuity. MedChemComm 4, 515–519 (2013).
Antermite, D., Degennaro, L. & Luisi, R. Recent advances in the chemistry of metallated azetidines. Org. Biomol. Chem. 15, 34–50 (2017).
Brandi, A., Cicchi, S. & Cordero, F. M. Novel syntheses of azetidines and azetidinones. Chem. Rev. 108, 3988–4035 (2008).
Cromwell, N. H. & Phillips, B. The azetidines. Recent synthetic developments. Chem. Rev. 79, 331–358 (1979).
Cox, B., Booker-Milburn, K. I., Elliott, L. D., Robertson-Ralph, M. & Zdorichenko, V. Escaping from flatland: [2+2] photocycloaddition; conformationally constrained sp3-rich scaffolds for lead generation. ACS Med. Chem. Lett. 10, 1512–1517 (2019).
Oderinde, M. S. et al. Synthesis of cyclobutane-fused tetracyclic scaffolds via visible-light photocatalysis for building molecular complexity. J. Am. Chem. Soc. 142, 3094–3103 (2020).
D’Auria, M. The Paternò–Büchi reaction—a comprehensive review. Photochem. Photobiol. Sci. 18, 2297–2362 (2019).
Poplata, S., Tröster, A., Zou, Y. Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2+2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Richardson, A. D., Becker, M. R. & Schindler, C. S. Synthesis of azetidines by aza Paternò–Büchi reactions. Chem. Sci. 11, 7553–7561 (2020).
Koch, T. H. & Howard, K. H. 2+2 Photocycloaddition to a carbon nitrogen double bond I. 3-ethoxyisoindolone. Tetrahedron Lett. 13, 4035–4038 (1972).
Howard, K. A. & Koch, T. H. Photochemical reactivity of keto imino ethers. V. (2+2) Photocycloaddition to the carbon–nitrogen double bond of 3-ethoxyisoindolone. J. Am. Chem. Soc. 97, 7288–7298 (1975).
Hyatt, J. A. & Swenton, J. S. Photochemical reactivity of 2,4-dimethyl-1,2,4-triazine-3,5-(2H)-dione (1,3-dimethyl-6-azauracil). J. Chem. Soc. Chem. Commun. 1972, 1144–1145 (1972).
Swenton, J. S. & Hyatt, J. A. Photosensitized cycloadditions to 1,3-dimethyl-6-azauracil and 1,3-dimethyl-6-azathymine. An imine linkage unusually reactive toward photocycloaddition. J. Am. Chem. Soc. 96, 4879–4885 (1974).
Koch, T. H., Higgins, R. H. & Schuster, H. F. An azetine from a photocycloaddition reaction followed by a retro Diels–Alder fragmentation. Tetrahedron Lett. 18, 431–434 (1977).
Futamura, S., Ohta, H. & Kamiya, Y. Photocycloaddition of 6-cyanophenanthridine to electron-rich olefins. Chem. Lett. 9, 655–658 (1980).
Kumagai, T., Shimizu, K., Kawamura, Y. & Mukai, T. Photochemistry of 3-aryl-2-isoxazoline. Tetrahedron 37, 3365–3376 (1981).
Kumagai, T., Shimizu, K., Kawamura, Y. & Mukai, T. Photocycloaddition of 3-aryl-2-isoxazolines with five-membered heterocycles. Chem. Lett. 12, 1357–1360 (1983).
Kawamura, Y., Kumagai, T. & Mukai, T. Photocycloaddition reaction of 3-aryl-2-isoxazolines with indene. Generation of [2+2] cycloadduct stereoisomers. Chem. Lett. 14, 1937–1940 (1985).
Nishio, T. The (2+2) photocycloaddition of the carbon–nitrogen double bond of quinoxalin-2(1H)-ones to electron-deficient olefins. J. Org. Chem. 49, 827–832 (1984).
Nishio, T. & Omote, Y. Photocycloaddition reactions of 1,4-benzoxazin-2-ones and electron-poor olefins. J. Org. Chem. 50, 1370–1373 (1985).
Declerck, V. & Aitken, D. J. N-Aminoazetidinecarboxylic acid: direct access to a small-ring hydrazino acid. J. Org. Chem. 76, 708–711 (2011).
Sampedro, D., Soldevilla, A., Campos, P. J., Ruiz, R. & Rodríguez, M. A. Regio- and stereochemistry of [2+2] photocycloadditions of imines to alkenes: a computational and experimental study. J. Org. Chem. 73, 8331–8336 (2008).
Sakamoto, R., Inada, T., Sakurai, S. & Maruoka, K. [2+2] Photocycloadditions between the carbon–nitrogen double bonds of imines and carbon–carbon double bonds. Org. Lett. 18, 6252–6255 (2016).
Kumarasamy, E., Kandappa, S. K., Raghunathan, R., Jockusch, S. & Sivaguru, J. Realizing an aza Paternò–Büchi reaction. Angew. Chem. Int. Ed. 56, 7056–7061 (2017).
Becker, M. R., Richardson, A. D. & Schindler, C. S. Functionalized azetidines via visible light-enabled aza Paternò–Büchi reactions. Nat. Commun. 10, 5095 (2019).
Lu, Z. & Yoon, T. P. Visible light photocatalysis of [2+2] styrene cycloadditions by energy transfer. Angew. Chem. Int. Ed. 51, 10329–10332 (2012).
Patra, T., Bellotti, P., Strieth-Kalthoff, F. & Glorius, F. Photosensitized intermolecular carboimination of alkenes through the persistent radical effect. Angew. Chem. Int. Ed. 59, 3172–3177 (2020).
Nicastri, M. C., Lehnherr, D., Lam, Y., DiRocco, D. A. & Rovis, T. Synthesis of sterically hindered primary amines by concurrent tandem photoredox catalysis. J. Am. Chem. Soc. 142, 987–998 (2020).
Roth, H. D. in PATAI’s Chemistry of Functional Groups 1–63 (ed. Marek, I.) (Wiley, 2010).
Strieth-Kalthoff, F., James, M. J., Teders, M., Pitzer, L. & Glorius, F. Energy transfer catalysis mediated by visible light: principles, applications, directions. Chem. Soc. Rev. 47, 7190–7202 (2018).
Carreira, E. M. & Fessard, T. C. Four-membered ring-containing spirocycles: synthetic strategies and opportunities. Chem. Rev. 114, 8257–8322 (2014).
Hughes, D. L. Patent review of manufacturing routes to recently approved oncology drugs: ibrutinib, cobimetinib, and alectinib. Org. Process Res. Dev. 20, 1855–1869 (2016).
Markham, A. Delafloxacin: first global approval. Drugs 77, 1481–1486 (2017).
Clark, J. D., Flanagan, M. E. & Telliez, J.-B. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J. Med. Chem. 57, 5023–5038 (2014).
Akihisa, T. et al. (+)- and (–)-syn-2-Isobutyl-4-methylazetidine-2,4-dicarboxylic acids from the extract of Monascus pilosus-fermented rice (red-mold rice). J. Nat. Prod. 67, 479–480 (2004).
Teegardin, K., Day, J. I., Chan, J. & Weaver, J. Advances in photocatalysis: a microreview of visible light mediated ruthenium and iridium catalyzed organic transformations. Org. Process Res. Dev. 20, 1156–1163 (2016).
Herkstroeter, W. G., Lamola, A. A. & Hammond, G. S. Mechanisms of photochemical reactions in solution. XXVIII. Values of triplet excitation energies of selected sensitizers. J. Am. Chem. Soc. 86, 4537–4540 (1964).
Acknowledgements
We thank J. W. Kampf for X-ray crystallographic studies. C.S.S. thanks the Alfred P. Sloan Foundation, the David and Lucile Packard Foundation and the Camille and Henry Dreyfus Foundation for fellowships. M.R.B. is thankful for a Peter A. S. Smith Endowment Award for research and a Rackham Predoctoral Fellowship. E.R.W. thanks the National Science Foundation for a predoctoral fellowship. We thank C. R. J. Stephenson for helpful discussions.
Author information
Authors and Affiliations
Contributions
M.R.B., E.R.W. and C.S.S. designed the experiments. M.R.B. and E.R.W. conducted and analysed the experiments described in this report. M.R.B., E.R.W. and C.S.S. prepared this manuscript for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–20, Tables 1–11, experimental procedures, new compound characterization data, X-ray crystallographic information and mechanistic experiments.
Supplementary Data 1
Crystallographic data for compound 59. CCDC reference 1980952.
Supplementary Data 2
Crystallographic data for compound 44. CCDC reference 1980947.
Supplementary Data 3
Crystallographic data for compound 46. CCDC reference 1980951.
Rights and permissions
About this article
Cite this article
Becker, M.R., Wearing, E.R. & Schindler, C.S. Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions. Nat. Chem. 12, 898–905 (2020). https://doi.org/10.1038/s41557-020-0541-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0541-1
This article is cited by
-
Accessing ladder-shape azetidine-fused indoline pentacycles through intermolecular regiodivergent aza-Paternò–Büchi reactions
Nature Communications (2024)
-
Photocatalytic Z/E isomerization unlocking the stereodivergent construction of axially chiral alkene frameworks
Nature Communications (2024)
-
The impact of UV light on synthetic photochemistry and photocatalysis
Nature Chemistry (2024)
-
Energy-transfer-induced [3+2] cycloadditions of N–N pyridinium ylides
Nature Chemistry (2023)
-
Diastereo- and atroposelective synthesis of N-arylpyrroles enabled by light-induced phosphoric acid catalysis
Nature Communications (2023)