Abstract
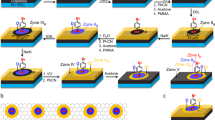
Chemical reactions that convert sp2 to sp3 hybridization have been demonstrated to be a fascinating yet challenging route to functionalize graphene. So far it has not been possible to precisely control the reaction sites nor their lateral order at the atomic/molecular scale. The application prospects have been limited for reactions that require long soaking, heating, electric pulses or probe-tip press. Here we demonstrate a spatially selective photocycloaddition reaction of a two-dimensional molecular network with defect-free basal plane of single-layer graphene. Directly visualized at the submolecular level, the cycloaddition is triggered by ultraviolet irradiation in ultrahigh vacuum, requiring no aid of the graphene Moiré pattern. The reaction involves both [2+2] and [2+4] cycloadditions, with the reaction sites aligned into a two-dimensional extended and well-ordered array, inducing a bandgap for the reacted graphene layer. This work provides a solid base for designing and engineering graphene-based optoelectronic and microelectronic devices.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The methods and materials used in this study are available in the Supplementary Information. The authors declare that all data supporting the findings of this study are available within the paper and its Supplementary Information. Correspondence and requests for materials should be addressed to M.Y, A.G., L.K or F. B.
References
Geim, A. K. Graphene: status and prospects. Science 324, 1530–1534 (2009).
Patera, L. L. et al. Real-time imaging of adatom-promoted graphene growth on nickel. Science 359, 1243–1246 (2018).
Schwierz, F. Graphene transistors. Nat. Nanotechnol. 5, 487–496 (2010).
Yan, L. et al. Chemistry and physics of a single atomic layer: strategies and challenges for functionalization of graphene and graphene-based materials. Chem. Soc. Rev. 41, 97–114 (2012).
Zhao, L. Y. et al. Visualizing individual nitrogen dopants in monolayer graphene. Science 333, 999–1003 (2011).
Ci, L. J. et al. Atomic layers of hybridized boron nitride and graphene domains. Nat. Mater. 9, 430–435 (2010).
Nair, M. N. et al. High van Hove singularity extension and Fermi velocity increase in epitaxial graphene functionalized by intercalated gold clusters. Phys. Rev. B 85, 245421–245426 (2012).
Bai, J. W. et al. Graphene nanomesh. Nat. Nanotechnol. 5, 190–194 (2010).
Treier, M. et al. Surface-assisted cyclodehydrogenation provides a synthetic route towards easily processable and chemically tailored nanographenes. Nat. Chem. 3, 61–67 (2011).
Palma, C. A. et al. Photoinduced C–C reactions on insulators toward photolithography of graphene nanoarchitectures. J. Am. Chem. Soc. 136, 4651–4658 (2014).
Narita, A. et al. Synthesis of structurally well-defined and liquid-phase-processable graphene nanoribbons. Nat. Chem. 6, 126–132 (2014).
Cai, J. M. et al. Graphene nanoribbon heterojunctions. Nat. Nanotechnol. 9, 896–900 (2014).
Chen, Y. C. et al. Molecular bandgap engineering of bottom-up synthesized graphene nanoribbon heterojunctions. Nat. Nanotechnol. 10, 156–160 (2015).
Han, P. et al. Self-assembly strategy for fabricating connected graphene nanoribbons. ACS Nano 9, 12035–12044 (2015).
Ruffieux, P. et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 531, 489–492 (2016).
Chen, Z. P. et al. Synthesis of graphene nanoribbons by ambient-pressure chemical vapor deposition and device integration. J. Am. Chem. Soc. 138, 15488–15496 (2016).
Nguyen, G. D. et al. Atomically precise graphene nanoribbon heterojunctions from a single molecular precursor. Nat. Nanotech. 12, 1077–1082 (2017).
Moreno, C. et al. Bottom-up synthesis of multifunctional nanoporous graphene. Science 360, 199–203 (2018).
Elias, D. C. et al. Control of graphene’s properties by reversible hydrogenation: evidence for graphane. Science 323, 610–613 (2009).
Balog, R. et al. Bandgap opening in graphene induced by patterned hydrogen adsorption. Nat. Mater. 9, 315–319 (2010).
Jørgensen, J. H. et al. Symmetry-driven band gap engineering in hydrogen functionalized graphene. ACS Nano 10, 10798–10807 (2016).
Li, H. et al. Site-selective local fluorination of graphene induced by focused ion beam irradiation. Sci. Rep. 6, 19719 (2016).
Altenburg, S. J., Lattelais, M., Wang, B., Bocquet, M. L. & Berndt, R. Reaction of phthalocyanines with graphene on Ir(111). J. Am. Chem. Soc. 137, 9452–9458 (2015).
Greenwood, J. et al. Covalent modification of graphene and graphite using diazonium chemistry: tunable grafting and nanomanipulation. ACS Nano 9, 5520–5535 (2015).
He, Y. Q. et al. Fusing tetrapyrroles to graphene edges by surface-assisted covalent coupling. Nat. Chem. 9, 33–38 (2017).
Daukiya, L. et al. Covalent functionalization by cycloaddition reactions of pristine defect-free graphene. ACS Nano 11, 627–634 (2017).
Criado, A., Melchionna, M., Marchesan, S. & Prato, M. The covalent functionalization of graphene on substrates. Angew. Chem. Int. Ed. 54, 10734–10750 (2015).
Bian, S. et al. Covalently patterned graphene surfaces by a force-accelerated Diels–Alder reaction. J. Am. Chem. Soc. 135, 9240–9243 (2013).
Cao, Y., Osuna, S., Liang, Y., Haddon, R. C. & Houk, K. N. Diels–Alder reactions of graphene: computational predictions of products and sites of reaction. J. Am. Chem. Soc. 135, 17643–17649 (2013).
Navarro, J. J. et al. Organic covalent patterning of nanostructured graphene with selectivity at the atomic level. Nano Lett. 16, 355–361 (2016).
Navarro, J. J., Calleja, F., Miranda, R. E., Pérez, M. & Vázquez de Parga, A. L. High yielding and extremely site-selective covalent functionalization of graphene. Chem. Commun. 53, 10418–10421 (2017).
Liu, L. H., Lerner, M. M. & Yan, M. Derivitization of pristine graphene with well-defined chemical functionalities. Nano Lett. 10, 3754–3756 (2010).
Denis, P. A. & Iribarne, F. Cooperative behavior in functionalized graphene: explaining the occurrence of 1,3 cycloaddition of azomethine ylides onto graphene. Chem. Phys. Lett. 550, 111–117 (2012).
MacLeod, J. M. & Rosei, F. Molecular self-assembly on graphene. Small 10, 1038–1049 (2014).
Kelly, R. E. A. & Kantorovich, L. Planar nucleic acid base super-structures. J. Mater. Chem. 16, 1984–1905 (2006).
Wang, Q. H. et al. Understanding and controlling the substrate effect on graphene electron-transfer chemistry via reactivity imprint lithography. Nat. Chem. 4, 724–732 (2012).
Khomyakov, P. A. et al. First-principles study of the interaction and charge transfer between graphene and metals. Phys. Rev. B 79, 195425–195436 (2009).
Laegsgaard, E. et al. A high-pressure scanning tunneling microscope. Rev. Sci. Instrum. 72, 3537–3542 (2001).
Shen, K. et al. Fabricating quasi-free-standing graphene on a SiC(0001) surface by steerable intercalation of iron. J. Phys. Chem. C 122, 21484–21492 (2018).
Kresse, G. et al. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P. et al. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S. et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H–Pu. J. Chem. Phys. 132, 154104 (2010).
Vande Vondele, J. et al. Quickstep: fast and accurate density functional calculations using a mixed Gaussian and plane waves approach. Comput. Phys. Commun. 167, 103–128 (2005).
Hutter, J. et al. CP2K: atomistic simulations of condensed matter systems. WIREs Comput. Mol. Sci. 4, 15–25 (2014).
Jónsson, H., Mills, G. & Jacobsen, K. W. in Classical and Quantum Dynamics in Condensed Phase Simulations (eds Berne, B. J., Ciccotti, G. & Coker, D. F.) 385–404 (World Scientific, 1998).
Henkelman, G. & Jonson, H. A climbing image nudged elastic band method finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant nos. 21473045, 51772066, 21603086, U1930402), the Engineering and Physical Sciences Research Council (grant nos. EP/L000202, EP/P020194), and the State Key Laboratory of Urban Water Resource and Environment (grant no. 2018DX04). We acknowledge X. Yang (Dalian Institute of Chemical Physics, Chinese Academy of Sciences) for the initial idea of triggering the reaction by ultraviolet irradiation and useful discussion. We also acknowledge the computational support from the Beijing Computational Science Research Center. F.R. acknowledges partial salary support from the Canada Research Chairs programme.
Author information
Authors and Affiliations
Contributions
A.G., M.Y. and F.B. designed the project. C.M. and A.G. synthesized the BCM molecule. C.C., G.S., S.W. and Z.L. conducted the graphene growth/BCM–graphene reaction/STM imaging/Raman analysis. W.H., C.C. and M.C. collected the IRAS results. K.S., F.S., Z.L. and J.P. carried out the ARPES studies. Q.L., L.K., H.S., P.D. and P.G. performed the calculations. M.Y., C.C., Y.S., M.C., F.R., L.K. and F. S. analysed and interpreted the results. M.Y., C.C., L.K. and F.R. wrote the manuscript. F.B. advised the project process.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Discussion and Tables 1–3.
Rights and permissions
About this article
Cite this article
Yu, M., Chen, C., Liu, Q. et al. Long-range ordered and atomic-scale control of graphene hybridization by photocycloaddition. Nat. Chem. 12, 1035–1041 (2020). https://doi.org/10.1038/s41557-020-0540-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0540-2
This article is cited by
-
Extending on-surface synthesis from 2D to 3D by cycloaddition with C60
Nature Communications (2023)
-
Oxygen-promoted synthesis of armchair graphene nanoribbons on Cu(111)
Science China Chemistry (2021)