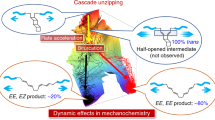
Abstract
Chemical reactions usually proceed through a radical, concerted or ionic mechanism; transformations in which all three mechanisms occur are rare. In polymer mechanochemistry, a mechanical force, transduced along polymer chains, is used to activate covalent bonds in mechanosensitive molecules (mechanophores). Cleavage of a C–C bond often follows a homolytic pathway, but some mechanophores have also been designed that react in a concerted or, more rarely, a heterolytic manner. Here, using 1H- and 19F-nuclear magnetic resonance spectroscopy in combination with deuterium labelling, we show that the dissociation of a mechanophore built around an N-heterocyclic carbene precursor proceeds with the rupture of a C–C bond through concomitant heterolytic, concerted and homolytic pathways. The distribution of products probably arises from a post-transition-state bifurcation in the reaction pathway, and their relative proportion is dictated by the polarization of the scissile C–C bond.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Crystallographic data for the compound 2H have been deposited at the Cambridge Crystallographic Data Centre under deposition number CCDC 1991781. The data that support the findings of this study are available within the paper and its Supplementary Information, or are available from the figshare data repository (https://doi.org/10.6084/m9.figshare.12156378.v1). Source data are provided with this paper.
References
Beyer, M. K. & Clausen-Schaumann, H. Mechanochemistry: the mechanical activation of covalent bonds. Chem. Rev. 105, 2921–2948 (2005).
Caruso, M. M. et al. Mechanically-induced chemical changes in polymeric materials. Chem. Rev. 109, 5755–5798 (2009).
Izak-Nau, E., Campagna, D., Baumann, C. & Göstl, R. Polymer mechanochemistry-enabled pericyclic reactions. Polym. Chem. 11, 2274–2299 (2020).
Klukovich, H. M. et al. Tension trapping of carbonyl ylides facilitated by a change in polymer backbone. J. Am. Chem. Soc. 134, 9577–9580 (2012).
Shiraki, T., Diesendruck, C. E. & Moore, J. S. The mechanochemical production of phenyl cations through heterolytic bond scission. Faraday Discuss. 170, 385–394 (2014).
Diesendruck, C. E. et al. Mechanically triggered heterolytic unzipping of a low-ceiling-temperature polymer. Nat. Chem. 6, 623–628 (2014).
Peterson, G. I. & Boydston, A. J. Kinetic analysis of mechanochemical chain scission of linear poly(phthalaldehyde). Macromol. Rapid Comm 35, 1611–1614 (2014).
Di Giannantonio, M. et al. Triggered metal ion release and oxidation: ferrocene as a mechanophore in polymers. Angew. Chem. Int. Ed. 57, 11445–11450 (2018).
Sha, Y. et al. Quantitative and mechanistic mechanochemistry in ferrocene dissociation. ACS Macro Lett. 10, 1174–1179 (2018).
Sha, Y. et al. Generalizing metallocene mechanochemistry to ruthenocene mechanophores. Chem. Sci. 10, 4959–4965 (2019).
Hickenboth, C. R. et al. Biasing reaction pathways with mechanical force. Nature 446, 423–427 (2007).
Wang, J. et al. Inducing and quantifying forbidden reactivity with single-molecule polymer mechanochemistry. Nat. Chem. 7, 323–327 (2015).
Lenhardt, J. M. et al. Trapping a diradical transition state by mechanochemical polymer extension. Science 329, 1057–1060 (2010).
Wang, J., Kouznetsova, T. B. & Craig, S. L. Reactivity and mechanism of a mechanically activated anti-Woodward–Hoffmann–DePuy reaction. J. Am. Chem. Soc. 137, 11554–11557 (2015).
Garcia-Manyes, S., Liang, J., Szoszkiewicz, R., Kuo, T.-L. & Fernández, J. M. Force-activated reactivity switch in a bimolecular chemical reaction. Nat. Chem. 1, 236–242 (2009).
Tian, Y., Kucharski, T. J., Yang, Q.-Z. & Boulatov, R. Model studies of force-dependent kinetics of multi-barrier reactions. Nat. Commun. 4, 2538 (2013).
Pill, M. F., East, A. L. L., Marx, D., Beyer, M. K. & Clausen-Schaumann, H. Mechanical activation drastically accelerates amide bond hydrolysis, matching enzyme activity. Angew. Chem. Int. Ed. 58, 9787–9790 (2019).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Sijbesma, R. P., Karthikeyan, S., Potisek, S. L. & Piermattei, A. Highly efficient mechanochemical scission of silver-carbene coordination polymers. J. Am. Chem. Soc. 130, 14968–14969 (2008).
Piermattei, A., Karthikeyan, S. & Sijbesma, R. P. Activating catalysts with mechanical force. Nat. Chem. 1, 133–137 (2009).
Michael, P. & Binder, W. H. A mechanochemically triggered ‘click’ catalyst. Angew. Chem. Int. Ed. 54, 13918–13922 (2015).
Clough, J. M., Balan, A., van Daal, T. L. J. & Sijbesma, R. P. Probing force with mechanobase-induced chemiluminescence. Angew. Chem. Int. Ed. 55, 1445–1449 (2016).
Flanigan, D. M., Romanov-Michailidis, F., White, N. A. & Rovis, T. Organocatalytic reactions enabled by N-heterocyclic carbenes. Chem. Rev. 115, 9307–9387 (2015).
Naumann, S. & Dove, A. P. N-Heterocyclic carbenes as organocatalysts for polymerizations: trends and frontiers. Polym. Chem. 6, 3185–3200 (2015).
Naumann, S. & Buchmeiser, M. R. Liberation of N-heterocyclic carbenes (NHCs) from thermally labile progenitors: protected NHCs as versatile tools in organo- and polymerization catalysis. Catal. Sci. Technol. 4, 2466–2479 (2014).
Nyce, G. W., Csihony, S., Waymouth, R. M. & Hedrick, J. L. A general and versatile approach to thermally generated N‐heterocyclic carbenes. Chem. Eur. J. 10, 4073–4079 (2004).
Beyer, M. The mechanical strength of a covalent bond calculated by density functional theory. J. Chem. Phys. 112, 7307–7312 (2000).
Dubois, P., Coulembier, O. & Raquez, J.-M. Handbook of Ring-Opening Polymerization (John Wiley & Sons, 2009).
Meldal, M. & Tornoe, C. W. Cu-catalyzed azide–alkyne cycloaddition. Chem. Rev. 108, 2952–3015 (2008).
Blum, A. P., Ritter, T. & Grubbs, R. H. Synthesis of N-heterocylic carbene-containing metal complexes from 2-(pentafluorophenyl)imidazolidines. Organometallics 26, 2122–2124 (2007).
Hare, S. R. & Tantillo, D. J. Post-transition state bifurcations gain momentum—current state of the field. Pure Appl. Chem. 89, 679–698 (2017).
Ess, D. H. et al. Bifurcations on potential energy surfaces of organic reactions. Angew. Chem. Int. Ed. 47, 7592–7601 (2008).
Chen, Z. et al. The cascade unzipping of ladderane reveals dynamic effects in mechanochemistry. Nat. Chem. 12, 302–309 (2020).
Wollenhaupt, M., Schran, C., Krupicka, M. & Marx, D. Force‐induced catastrophes on energy landscapes: mechanochemical manipulation of downhill and uphill bifurcations explains the ring‐opening selectivity of cyclopropanes. ChemPhysChem 19, 837–847 (2018).
Acknowledgements
We thank the EPSRC for giving a studentship to R.N. and the Royal Society for giving a University Research Fellowship to G.D.B.
Author information
Authors and Affiliations
Contributions
G.D.B. conceived the project. R.N. and G.D.B. designed the experiments. R.N. carried out the experimental work. G.D.B performed the calculations. All the authors contributed to the analysis of the results and the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental procedures, NMR spectra and computational details.
Supplementary Data
Crystallographic data for the compound 2H, CCDC 1991781.
Source data
Source Data Fig. 6
Source data of PES in Fig. 6b.
Rights and permissions
About this article
Cite this article
Nixon, R., De Bo, G. Three concomitant C–C dissociation pathways during the mechanical activation of an N-heterocyclic carbene precursor. Nat. Chem. 12, 826–831 (2020). https://doi.org/10.1038/s41557-020-0509-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0509-1
This article is cited by
-
Azobenzene as a photoswitchable mechanophore
Nature Chemistry (2024)
-
Strong ferromagnetism of g-C3N4 achieved by atomic manipulation
Nature Communications (2023)
-
The many flavours of mechanochemistry and its plausible conceptual underpinnings
Nature Reviews Chemistry (2021)