Abstract
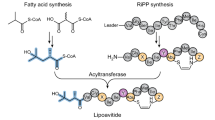
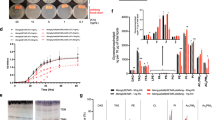
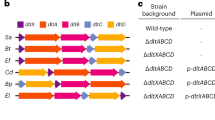
Fusions of fatty acids and peptides expand the structural diversity of natural products; however, polyketide/ribosomally synthesized and post-translationally modified peptides (PK/RiPPs) hybrid lipopeptides are relatively rare. Here we report a family of PK/RiPPs called goadvionins, which inhibit the growth of Gram-positive bacteria, and an acyltransferase, GdvG, which catalyses the condensation of the PK and RiPP moieties. Goadvionin comprises a trimethylammonio 32-carbon acyl chain and an eight-residue RiPP with an avionin structure. The positions of six hydroxyl groups and one double bond in the very-long acyl chain were determined by radical-induced dissociation tandem mass spectrometry, which collides radical ion species to generate C–C bond cleavage fragments. GdvG belongs to the Gcn5-related N-acetyltransferase superfamily. Unlike conventional acyltransferases, GdvG transfers a very long acyl chain that is tethered to an acyl carrier protein to the N-terminal amino group of the RiPP moiety. gdvG homologues flanked by PK/fatty acid and RiPP biosynthesis genes are widely distributed in microbial species, suggesting that acyltransferase-catalysed condensation of PKs and RiPPs is a general strategy in biosynthesis of similar lipopeptides.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data are included in the manuscript and the Supplementary Information. The nucleotide sequence of the gdv cluster has been deposited in the DDBJ with accession number LC481990.
References
Baltz, R. H., Miao, V. & Wrigley, S. K. Natural products to drugs: daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 22, 717–741 (2005).
Arima, K., Kakinuma, A. & Tamura, G. Surfactin, a crystalline peptidelipid surfactant produced by Bacillus subtilis: isolation, characterization and its inhibition of fibrin clot formation. Biochem. Biophys. Res. Commun. 31, 488–494 (1968).
Choi, S. K. et al. Identification of a polymyxin synthetase gene cluster of Paenibacillus polymyxa and heterologous expression of the gene in Bacillus subtilis. J Bacteriol. 191, 3350–3358 (2009).
Marahiel, M. A. Protein templates for the biosynthesis of peptide antibiotics. Chem. Biol. 4, 561–567 (1997).
Duitman, E. H. et al. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl Acad. Sci. USA 96, 13294–13299 (1999).
Wiebach, V. et al. The anti-staphylococcal lipolanthines are ribosomally synthesized lipopeptides. Nat. Chem. Biol. 14, 652–654 (2018).
Aszodi, J., Carniato, D., Le Beller, D., Lesquame, G. & Quernin, M.-H. New bicyclic lipopeptide, preparation and use as antimicrobial agent. European Union patent EP15306065 (2015).
Favrot, L., Blanchard, J. S. & Vergnolle, O. Bacterial GCN5-related N-acetyltransferases: from resistance to regulation. Biochemistry 55, 989–1002 (2016).
Onaka, H., Nakaho, M., Hayashi, K., Igarashi, Y. & Furumai, T. Cloning and characterization of the goadsporin biosynthetic gene cluster from Streptomyces sp. TP-A0584. Microbiology 151, 3923–3933 (2005).
Ozaki, T. et al. Dissection of goadsporin biosynthesis by in vitro reconstitution leading to designer analogues expressed in vivo. Nat. Commun. 8, 14207 (2017).
Kupke, T., Stevanovic, S., Sahl, H. G. & Gotz, F. Purification and characterization of EpiD, a flavoprotein involved in the biosynthesis of the lantibiotic epidermin. J. Bacteriol. 174, 5354–5361 (1992).
Müller, W. M., Schmiederer, T., Ensle, P. & Süssmuth, R. D. In vitro biosynthesis of the prepeptide of type-III lantibiotic labyrinthopeptin A2 including formation of a C-C bond as a post-translational modification. Angew. Chem. Int. Ed. 49, 2436–2440 (2010).
Blin, K. et al. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, gkz310 (2019).
Kihara, A. Very long-chain fatty acids: elongation, physiology and related disorders. J. Biochem. 152, 387–395 (2012).
Wyche, T. P., Hou, Y. P., Vazquez-Rivera, E., Braun, D. & Bugni, T. S. Peptidolipins B-F, antibacterial lipopeptides from an ascidian-derived Nocardia sp. J. Nat. Prod. 75, 735–740 (2012).
Ekroos, K. et al. Charting molecular composition of phosphatidylcholines by fatty acid scanning and ion trap MS3 fragmentation. J. Lipid Res. 44, 2181–2192 (2003).
Takahashi, H. et al. Hydrogen attachment/abstraction dissociation (HAD) of gas-phase peptide ions for tandem mass spectrometry. Anal. Chem. 88, 3810–3816 (2016).
Takahashi, H. et al. Structural analysis of phospholipid using hydrogen abstraction dissociation and oxygen attachment dissociation in tandem mass spectrometry. Anal. Chem. 90, 7230–7238 (2018).
Noike, M. et al. A peptide ligase and the ribosome cooperate to synthesize the peptide pheganomycin. Nat. Chem. Biol. 11, 71–76 (2015).
Chooi, Y. H. & Tang, Y. Adding the lipo to lipopeptides: do more with less. Chem. Biol. 17, 791–793 (2010).
Cosmina, P. et al. Sequence and analysis of the genetic locus responsible for surfactin synthesis in Bacillus subtilis. Mol. Microbiol. 8, 821–831 (1993).
Kraas, F. I., Helmetag, V., Wittmann, M., Strieker, M. & Marahiel, M. A. Functional dissection of surfactin synthetase initiation module reveals insights into the mechanism of lipoinitiation. Chem. Biol. 17, 872–880 (2010).
Towler, D. A. et al. Myristoyl Coa-protein N-myristoyltransferase activities from rat-liver and yeast possess overlapping yet distinct peptide substrate specificities. J. Biol. Chem. 263, 1784–1790 (1988).
Van Wagoner, R. M. & Clardy, J. FeeM, an N-acyl amino acid synthase from an uncultured soil microbe: structure, mechanism, and acyl carrier protein binding. Structure 14, 1425–1435 (2006).
Hiratsuka, T. et al. Biosynthesis of the structurally unique polycyclopropanated polyketide-nucleoside hybrid jawsamycin (FR-900848). Angew. Chem. Int. Ed. 53, 5423–5426 (2014).
Gu, L. et al. GNAT-like strategy for polyketide chain initiation. Science 318, 970–974 (2007).
Forouhar, F. et al. Structural and functional evidence for Bacillus subtilis PaiA as a novel N1-spermidine/spermine acetyltransferase. J Biol. Chem. 280, 40328–40336 (2005).
Peddie, V. et al. Cytotoxic glycosylated fatty acid amides from a Stelletta sp. marine sponge. J. Nat. Prod. 78, 2808–2813 (2015).
Nakao, Y., Maki, T., Matsunaga, S., van Soest, R. W. M. & Fusetani, N. Penarolide sulfates A(1) and A(2), new α-glucosidase inhibitors from a marine sponge Penares sp. Tetrahedron 56, 8977–8987 (2000).
Takada, K. et al. Schulzeines A-C, new α-glucosidase inhibitors from the marine sponge Penares schulzei. J. Am. Chem. Soc. 126, 187–193 (2004).
Konno, K., Shirahama, H. & Matsumoto, T. Clithioneine, an amino-acid betaine from Clitocybe acromelalga. Phytochemistry 23, 1003–1006 (1984).
Ghosal, S. & Srivastava, R. S. Structure of erysophorine: a new quaternary alkaloid of Erythrina arborescens. Phytochemistry 13, 2603–2605 (1974).
Parkinson, E. I. et al. Discovery of the tyrobetaine natural products and their biosynthetic gene cluster via metabologenomics. ACS Chem. Biol. 13, 1029–1037 (2018).
Smith, T. E. et al. Accessing chemical diversity from the uncultivated symbionts of small marine animals. Nat. Chem. Biol. 14, 179–185 (2018).
Julien, B., Tian, Z. Q., Reid, R. & Reeves, C. D. Analysis of the ambruticin and jerangolid gene clusters of Sorangium cellulosum reveals unusual mechanisms of polyketide biosynthesis. Chem. Biol. 13, 1277–1286 (2006).
Matsuo, Y., Ishida, R., Matsumoto, T., Tatewaki, M. & Suzuki, M. Yendolipin, a novel lipobetaine with an inhibitory activity toward morphogenesis in a foliaceous green alga Monostroma oxyspermum. Tetrahedron 53, 869–876 (1997).
Onaka, H., Taniguchi, S., Ikeda, H., Igarashi, Y. & Furumai, T. pTOYAMAcos, pTYM18, and pTYM19, actinomycete Escherichia coli integrating vectors for heterologous gene expression. J. Antibiot. (Tokyo) 56, 950–956 (2003).
Ishikawa, J. & Hotta, K. FramePlot: a new implementation of the frame analysis for predicting protein-coding regions in bacterial DNA with a high G + C content. FEMS Microbiol. Lett. 174, 251–253 (1999).
Komatsu, M. et al. Engineered Streptomyces avermitilis host for heterologous expression of biosynthetic gene cluster for secondary metabolites. ACS Synth. Biol. 2, 384–396 (2013).
Onaka, H., Tabata, H., Igarashi, Y., Sato, Y. & Furumai, T. Goadsporin, a chemical substance which promotes secondary metabolism and morphogenesis in streptomycetes. I. Purification and characterization. J. Antibiot. (Tokyo) 54, 1036–1044 (2001).
Acknowledgements
This research was supported in part by a grant-in-aid from the IFO, Institute for Fermentation, Osaka (to H.O., S.A. and T. Ozaki), Amano Enzyme (to H.O and S.A), JSPS KAKENHI grant-in-aid for Young Scientists B (no. 26850044 to S.A.), JSPS KAKENHI grant-in-aid for Scientific Research on Innovative Areas (no. JP16H06444) and the JSPS A3 Foresight Program (to H.O., S.A. and Y.K.). We thank D. Du and T. Tsunoda for assistance with the in vitro assays, S. Kawano for assistance with genetic work, D. Asakawa for HAD-MS/MS data analysis, M. Wada and Y. Shimabukuro for HAD-MS/MS development, and Y. Ohnishi and S. Iwamoto for valuable comments on the manuscript.
Author information
Authors and Affiliations
Contributions
R.K. and Y.K. performed in vitro assays. T. Ozaki, T. Ono, S.A. and H.O. performed genome mining and bioinformatic analyses. S.H., H.T., Y.S., T. Ono, Kazuya Teramoto, Kanae Teramoto, Koichi Tanaka and I.A. determined chemical structures. R.K., S.H., H.T., Y.K., S.A., Koichi Tanaka and H.O. analysed the data. S.H., Y.K., and H.O. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–56, Methods and Tables 1–16.
Rights and permissions
About this article
Cite this article
Kozakai, R., Ono, T., Hoshino, S. et al. Acyltransferase that catalyses the condensation of polyketide and peptide moieties of goadvionin hybrid lipopeptides. Nat. Chem. 12, 869–877 (2020). https://doi.org/10.1038/s41557-020-0508-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0508-2
This article is cited by
-
Non-modular fatty acid synthases yield distinct N-terminal acylation in ribosomal peptides
Nature Chemistry (2024)
-
Molecular basis for carrier protein-dependent amide bond formation in the biosynthesis of lincosamide antibiotics
Nature Catalysis (2023)
-
Amino acid (acyl carrier protein) ligase-associated biosynthetic gene clusters reveal unexplored biosynthetic potential
Molecular Genetics and Genomics (2023)
-
Heterologous Production and Structure Determination of a New Lanthipeptide Sinosporapeptin Using a Cryptic Gene Cluster in an Actinobacterium Sinosporangium siamense
Journal of Microbiology (2023)
-
Computational mass spectrometry accelerates C = C position-resolved untargeted lipidomics using oxygen attachment dissociation
Communications Chemistry (2022)