Abstract
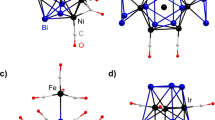
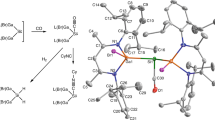
A variety of homoleptic transition metal carbonyl complexes are known as bulk compounds for group 7–12 metals. These metals typically feature a maximum of 6 CO ligands to form complexes with 18 valence electrons. In contrast, group 3–5 metals, with fewer valence electrons, have been shown to form highly coordinated heptacarbonyl and octacarbonyl complexes—although they were only identified by gas-phase mass spectrometry and/or matrix isolation spectroscopy work. Now we have prepared heptacarbonyl cations of niobium and tantalum as crystalline salts that are stable at room temperature. The [M(CO)7]+ (M = Nb or Ta) complexes were formed by the oxidation of [M(CO)6]– with 2Ag+[Al(ORF)4]– (RF, C(CF3)3) under a CO atmosphere; their experimental characterization was supported by density functional theory calculations. Other unusual carbonyl compounds were also synthesized: two isostructural salts that contained the 84-valence-electron cluster cation [Ag6{Nb(CO)6}4]2+, the piano-stool complexes [(1,2-F2C6H4)M(CO)4]+ and two polymorphs of neutral Ta2(CO)12 with a long, unsupported Ta–Ta bond.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Atomic coordinates and structure factors for the crystal structure of 1–6 are deposited at the Cambridge Crystallographic Data Centre (CCDC) under the accession codes 1909275 (1), 1909276 (2), 1909277 (3a), 1909279 (3b), 1909278 (4), 1909449 (5), 1909450 (6a) and 1909274 (6b); copies of the data can be obtained from www.ccdc.cam.ac.uk/data_request/cif. All the other data generated or analysed during this study are included in this published article (and its Supplementary Information files), and are available from the corresponding authors on reasonable request.
References
Mond, L., Langer, C. & Quincke, F. L.—Action of carbon monoxide on nickel. J. Chem. Soc. Trans. 57, 749–753 (1890).
Dyson, P. J. Catalysis by low oxidation state transition metal (carbonyl) clusters. Coord. Chem. Rev. 248, 2443–2458 (2004).
Kondo, T., Suzuki, N., Okada, T. & Mitsudo, T. First ruthenium-catalyzed intramolecular Pauson−Khand reaction. J. Am. Chem. Soc. 119, 6187–6188 (1997).
Motterlini, R. et al. Carbon monoxide-releasing molecules. Circ. Res. 90, e17–e24 (2002).
Romão, C. C., Blättler, W. A., Seixas, J. D. & Bernardes, G. J. L. Developing drug molecules for therapy with carbon monoxide. Chem. Soc. Rev. 41, 3571–3583 (2012).
Motterlini, R. & Otterbein, L. E. The therapeutic potential of carbon monoxide. Nat. Rev. 9, 728–743 (2010).
Tsumori, N., Xu, Q., Souma, Y. & Mori, H. Carbonylation of alcohols over Nafion-H, a solid perfluoroalkanesulfonic acid resin catalyst. J. Mol. Cat. A 179, 271–277 (2002).
Braunstein, P. & Naud, F. Hemilability of hybrid ligands and the coordination chemistry of oxazoline-based systems. Angew. Chem. Int. Ed. 40, 680–699 (2001).
Weber, L. et al. Organometallic chemistry of homoleptic carbonylmetal cations. 1. Stereospecific tetramerization of 2-propynol and polymerization of arylacetylenes by means of [Pt(CO)4][Sb2F11]2. Organometallics 18, 2497–2504 (1999).
Xu, Q. Metal carbonyl cations. Generation, characterization and catalytic application. Coord. Chem. Rev. 231, 83–108 (2002).
Semmelhack, M. F. & Clark, G. Meta-substituted aromatics by carbanion attack on π-anisole and π-toluenechromium tricarbonyl. J. Am. Chem. Soc. 99, 1675–1676 (1977).
Semmelhack, M. F., Bisaha, J. & Czarny, M. Metalation of arenechromium tricarbonyl complexes and electrophilic trapping of the complexed phenyllithium intermediate. J. Am. Chem. Soc. 101, 768–770 (1979).
Rose-Munch, F., Gagliardini, V., Renard, C. & Rose, E. (η6-Arene)tricarbonylchromium and (η5-cyclohexadienyl) tricarbonylmanganese complexes. Indirect nucleophilic substitutions. Coord. Chem. Rev. 178–180, 249–268 (1998).
Wu, X. et al. Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals. Science 361, 912–916 (2018).
Landis, C. R., Hughes, R. P. & Weinhold, F. Comment on “Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals”. Science 365, eaay2355 (2019).
Zhao, L., Pan, S., Zhou, M. & Frenking, G. Response to Comment on “Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals”. Science 365, eaay5021 (2019).
Bernhardt, E. et al. D 3d ground-state structure of V(CO)6: a combined matrix isolation and ab initio study of the Jahn−Teller effect. J. Phys. Chem. A 107, 859–868 (2003).
Willner, H. & Aubke, F. σ-Bonded metal carbonyl cations and their derivatives. Syntheses and structural, spectroscopic, and bonding principles. Organometallics 22, 3612–3633 (2003).
Frenking, G. Understanding the nature of the bonding in transition metal complexes: from Dewar’s molecular orbital model to an energy partitioning analysis of the metal–ligand bond. J. Organomet. Chem. 635, 9–23 (2001).
Frenking, G., Loschen, C., Krapp, A., Fau, S. & Strauss, S. H. Electronic structure of CO—an exercise in modern chemical bonding theory. J. Comput. Chem. 28, 117–126 (2007).
Lupinetti, A. J., Frenking, G. & Strauss, S. H. Nonclassical metal carbonyls: appropriate definitions with a theoretical justification. Angew. Chem. Int. Ed. 37, 2113–2116 (1998).
Willner, H. & Aubke, F. Homoleptic metal carbonyl cations of the electron-rich metals. Their generation in superacid media together with their spectroscopic and structural characterization. Angew. Chem. Int. Ed. Engl. 36, 2402–2425 (1997).
Hurlburt, P. K. et al. Nonclassical metal carbonyls: [Ag(CO)]+ and [Ag(CO)2]+. J. Am. Chem. Soc. 116, 10003–10014 (1994).
Lupinetti, A. J., Havighurst, M. D., Miller, S. M., Anderson, O. P. & Strauss, S. H. Facile Conversion of [(η6-C6H6)Rh(CO)2][1-Et-CB11F11] into the nonclassical rhodium(i) carbonyl [Rh(CO)4][1-Et-CB11F11]. J. Am. Chem. Soc. 121, 11920–11921 (2019).
Bistoni, G. et al. How π back-donation quantitatively controls the CO stretching response in classical and non-classical metal carbonyl complexes. Chem. Sci. 7, 1174–1184 (2016).
Ricks, A. M., Reed, Z. D. & Duncan, M. A. Seven-coordinate homoleptic metal carbonyls in the gas phase. J. Am. Chem. Soc. 131, 9176–9177 (2009).
Brathwaite, A. D., Maner, J. A. & Duncan, M. A. Testing the limits of the 18-electron rule: the gas-phase carbonyls of Sc+ and Y+. The gas-phase carbonyls of Sc+ and Y+. Inorg. Chem. 53, 1166–1169 (2014).
Jin, J. et al. Octacarbonyl anion complexes of group three transition metals [TM(CO)8]– (TM = Sc, Y, La) and the 18-electron rule. Angew. Chem. Int. Ed. 57, 6236–6241 (2018).
Bohnenberger, J. et al. Stable salts of the hexacarbonyl chromium(i) cation and its pentacarbonyl-nitrosyl chromium(i) analogue. Nat. Commun. 10, 624 (2019).
Xu, Q. et al. Hexacarbonyldiplatinum(i). Synthesis, spectroscopy, and density functional calculation of the first homoleptic, dinuclear platinum(i) carbonyl cation, [{Pt(CO)3}2]2+, formed in concentrated sulfuric acid. J. Am. Chem. Soc. 122, 6862–6870 (2000).
Malinowski, P. J. & Krossing, I. Ag[Fe(CO)5]2+: a bare silver complex with Fe(CO)5 as a ligand. Angew. Chem. Int. Ed. 53, 13460–13462 (2014).
Krossing, I. & Raabe, I. Noncoordinating anions—fact or fiction? A survey of likely candidates. Angew. Chem. Int. Ed. 43, 2066–2090 (2004).
Riddlestone, I. M., Kraft, A., Schaefer, J. & Krossing, I. Taming the cationic beast. Novel developments in the synthesis and application of weakly coordinating anions. Angew. Chem. Int. Ed. 57, 13982–14024 (2018).
Ivanova, S. M. et al. Mono-, di-, tri-, and tetracarbonyls of copper(i), including the structures of Cu(CO)2(1-Bn-CB11F11) and [Cu(CO)4][1-Et-CB11F11]. Inorg. Chem. 38, 3756–3757 (1999).
Bernhardt, E., Finze, M., Willner, H., Lehmann, C. W. & Aubke, F. Co(CO)5(CF3)3BF: a stable salt of a homoleptic trigonal–bipyramidal metal–carbonyl cation. Angew. Chem. Int. Ed. 42, 2077–2079 (2003).
Rodgers, M. T. & Armentrout, P. B. Cationic noncovalent interactions. Energetics and periodic trends. Chem. Rev. 116, 5642–5687 (2016).
Duncan, M. A. Infrared spectroscopy to probe structure and dynamics in metal ion–molecule complexes. Int. Rev. Phys. Chem. 22, 407–435 (2003).
Meier, S. C., Himmel, D. & Krossing, I. How does the environment influence a given cation? A systematic investigation of Co(CO)5 + in gas phase, solution, and solid state. Chem. Eur. J. 24, 19348–19360 (2018).
Krossing, I. The facile preparation of weakly coordinating anions: structure and characterisation of silverpolyfluoroalkoxyaluminates AgAl(ORF)4, calculation of the alkoxide ion affinity. Chem. Eur. J. 7, 490–502 (2001).
Dewey, C. G., Ellis, J. E., Fjare, K. L., Pfahl, K. M. & Warnock, G. F. P. A facile atmospheric pressure synthesis of the hexacarbonylmetalate ions, M(CO)6 –, of niobium and tantalum. Organometallics 2, 388–391 (1983).
Herrmann, W. A. 100 years of metal carbonyls. A serendipitous chemical discovery of major scientific and industrial impact. J. Organomet. Chem. 383, 21–44 (1990).
Calderazzo, F., Castellani, M., Pampaloni, G. & Zanazzi, P. F. New carbonyl derivatives of niobium(i) and tantalum(i). J. Chem. Soc. Dalton Trans. 1985, 1989–1995 (1985).
Yan, J. et al. Asymmetric synthesis of chiral bimetallic [Ag28Cu12(SR)24]4– nanoclusters via ion pairing. J. Am. Chem. Soc. 138, 12751–12754 (2016).
Hailmann, M. et al. Unprecedented efficient structure controlled phosphorescence of silver(i) clusters stabilized by carba-closo-dodecaboranylethynyl ligands. Angew. Chem. Int. Ed. Engl. 55, 10507–10511 (2016).
Jin, S. et al. Crystal structure and optical properties of the [Ag62S12(SBut)32]2+ nanocluster with a complete face-centered cubic kernel. J. Am. Chem. Soc. 136, 15559–15565 (2014).
Reiß, P., Weigend, F., Ahlrichs, R. & Fenske, D. [{Ag(tBuNH2)2}4][{Ag(tBuNH2)(tBuN=CHCH3)}2][Ag12(CF3CO2)14]: a compound with an Ag128 + cluster core. Angew. Chem. Int. Ed. 39, 3925–3929 (2000).
Calderazzo, F., Pampaloni, G., Englert, U. & Strähle, J. Electron-transfer processes with substituted group 5 metal carbonyls. Synthesis, crystal and molecular structure of Ag3M3(CO)12(Me2PCH2CH2PMe2)3, M = Nb, Ta, the first structurally characterized carbonyl derivatives of niobium(0) and tantalum(0). J. Organomet. Chem. 383, 45–57 (1990).
Tang, L. et al. The remarkable Nb2(CO)12 with seven-coordinate niobium: decarbonylation to Nb2(CO)11 and Nb2(CO)10. J. Chem. Theory Comput. 7, 2112–2125 (2011).
Sietzen, M., Wadepohl, H. & Ballmann, J. Synthesis and reactivity of cyclometalated triamidophosphine complexes of niobium and tantalum. Inorg. Chem. 54, 4094–4103 (2015).
Belmonte, P. A., Schrock, R. R. & Day, C. S. Binuclear tantalum hydride complexes. J. Am. Chem. Soc. 104, 3082–3089 (1982).
Miller, R. L. et al. Syntheses, carbonylations, and dihydrogen exchange studies of monomeric and dimeric silox (tert-Bu3SiO–) hydrides of tantalum: structure of [(silox)2TaH2]2. J. Am. Chem. Soc. 115, 5570–5588 (1993).
Scoles, L., Ruppa, K. B. P. & Gambarotta, S. Preparation of the first ditantalum(iii) complex containing a Ta−Ta bond without bridging ligands. J. Am. Chem. Soc. 118, 2529–2530 (1996).
Pyykkö, P. & Atsumi, M. Molecular double-bond covalent radii for elements Li–E112. Chem. Eur. J. 15, 12770–12779 (2009).
Batsanov, S. S. Van der Waals radii of elements. Inorg. Mat. 37, 871–885 (2001).
Assefa, M. K., Devera, J. L., Brathwaite, A. D., Mosley, J. D. & Duncan, M. A. Vibrational scaling factors for transition metal carbonyls. Chem. Phys. Lett. 640, 175–179 (2015).
Author information
Authors and Affiliations
Contributions
I.K. supervised the project. W.U. designed and performed the experiments and all the characterizations. M.S. and D.H. carried out the general quantum chemical calculations. W.U. and D.K. performed the single-crystal determination and refinement. All the authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Methods, syntheses of all compounds, vibrational, NMR and UV–vis analysis, crystallographic data and computational details.
Crystallographic Data
CIF for compound 1; CCDC reference: 1909275.
Crystallographic Data
CIF for compound 2; CCDC reference: 1909276.
Crystallographic Data
CIF for compound 3a; CCDC reference: 1909277.
Crystallographic Data
CIF for compound 3b; CCDC reference: 1909279.
Crystallographic Data
CIF for compound 4; CCDC reference: 1909278.
Crystallographic Data
CIF for compound 5; CCDC reference: 1909449.
Crystallographic Data
CIF for compound 6a; CCDC reference: 1909450.
Crystallographic Data
CIF for compound 6b; CCDC reference: 1909274.
Rights and permissions
About this article
Cite this article
Unkrig, W., Schmitt, M., Kratzert, D. et al. Synthesis and characterization of crystalline niobium and tantalum carbonyl complexes at room temperature. Nat. Chem. 12, 647–653 (2020). https://doi.org/10.1038/s41557-020-0487-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0487-3