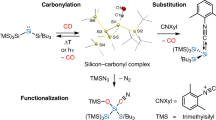
Abstract
Main-group-element compounds with energetically high-lying donor and low-lying acceptor orbitals are able to mimic chemical bonding motifs and reactivity patterns known in transition metal chemistry, including small-molecule activation and catalytic reactions. Monovalent group 13 compounds and divalent group 14 compounds, particularly silylenes, have been shown to be excellent candidates for this purpose. However, one of the most common reactions of transition metal complexes, the direct reaction with carbon monoxide and formation of room-temperature isolable carbonyl complexes, is virtually unknown in main-group-element chemistry. Here, we show the synthesis, single-crystal X-ray structure, and density functional theory computations of a room-temperature-stable silylene carbonyl complex [L(Br)Ga]2Si:–CO (L = HC[C(Me)N(2,6-iPr2-C6H3)]2), which was obtained by direct carbonylation of the electron-rich silylene intermediate [L(Br)Ga]2Si:. Furthermore, [L(Br)Ga]2Si:–CO reacts with H2 and PBr3 with bond activation, whereas the reaction with cyclohexyl isocyanide proceeds with CO substitution.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this Article (and its Supplementary Information). The structures of 1–6 in the solid state were determined by single-crystal X-ray diffraction and the crystallographic data have been deposited with the Cambridge Crystallographic Data Centre under nos. CCDC 1943186 (1), 1943187 (2), 1943188 (3), 1943189 (4), 1943190 (5) and 1967024 (6). Copies of the data can be obtained free of charge on application to CCDC.
References
Power, P. P. Main-group elements as transition metals. Nature 463, 171–177 (2010).
Martin, D., Soleilhavoup, M. & Bertrand, G. Stable singlet carbenes as mimics for transition metal centers. Chem. Sci. 2, 389–399 (2011).
Hansmann, M. M. & Bertrand, G. Transition-metal-like behavior of main group elements: ligand exchange at a phosphinidene. J. Am. Chem. Soc. 138, 15885–15888 (2016).
Weetman, C. & Inoue, S. The road travelled: after main-group elements as transition metals. ChemCatChem 10, 4213–4228 (2018).
Melen, R. L. Frontiers in molecular p-block chemistry: from structure to reactivity. Science 363, 479–484 (2019).
Leitao, E. M., Jurca, T. & Manners, I. Catalysis in service of main group chemistry offers a versatile approach to p-block molecules and materials. Nat. Chem. 5, 817–829 (2013).
Wu, X. et al. Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals. Science 361, 912–916 (2018).
Wu, X. et al. Barium as honorary transition metal in action: experimental and theoretical study of Ba(CO)+ and Ba(CO)−. Angew. Chem. Int. Ed. 57, 3974–3980 (2018).
Finze, M. et al. Tris(trifluoromethyl)borane carbonyl, (CF3)3BCO—synthesis, physical, chemical and spectroscopic properties, gas phase and solid state structure. J. Am. Chem. Soc. 124, 15385–15398 (2002).
Glore, J. D., Rathke, J. W. & Schaeffer, R. Some reactions of triborane(7) and the structure of triborane(7) carbonyl. Inorg. Chem. 12, 2175–2178 (1973).
Braunschweig, H. et al. Multiple complexation of CO and related ligands to a main-group element. Nature 522, 327–330 (2015).
Grant, L. N. et al. A planar Ti2P2 core assembled by reductive decarbonylation of −O−C≡P and P−P radical coupling. Chem. Eur. J. 23, 6272–6276 (2017).
Puschmann, F. F. et al. Phosphination of carbon monoxide: a simple synthesis of sodium phosphaethynolate (NaOCP). Angew. Chem. Int. Ed. 50, 8420–8423 (2011).
Hansmann, M. M., Jazzar, R. & Bertrand, G. Singlet (phosphino)phosphinidenes are electrophilic. J. Am. Chem. Soc. 138, 8356–8359 (2016).
Böhnke, J. et al. The synthesis of B2(SIDip)2 and its reactivity between the diboracumulenic and diborynic extremes. Angew. Chem. Int. Ed. 54, 13801–13805 (2015).
Braunschweig, H. et al. Metal-free binding and coupling of carbon monoxide at a boron–boron triple bond. Nat. Chem. 5, 1025–1029 (2013).
Arrowsmith, M., Böhnke, J., Braunschweig, H. & Celik, M. A. Reactivity of a dihydrodiborene with CO: coordination, insertion, cleavage and spontaneous formation of a cyclic alkyne. Angew. Chem. Int. Ed. 56, 14287–14292 (2017).
Zhang, H., Cao, Z., Wu, W. & Mo, Y. The transition-metal-like behavior of B2(NHC)2 in the activation of CO: HOMO–LUMO swap without photoinduction. Angew. Chem. Int. Ed. 57, 13076–13081 (2018).
Majumdar, M. et al. Reductive cleavage of carbon monoxide by a disilenide. Angew. Chem. Int. Ed. 54, 8746–8750 (2015).
Stephan, D. W. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 (2016).
Dobrovetsky, R. & Stephan, D. W. Stoichiometric metal-free reduction of CO in syn-gas. J. Am. Chem. Soc. 135, 4974–4977 (2013).
Devillard, M., de Bruin, B., Siegler, M. A. & van der Vlugt, J. I. Transition-metal-free cleavage of CO. Chem. Eur. J. 23, 13628–13632 (2017).
Lembke, R. R., Ferrante, R. F. & Weltner, W. Jr. SiCO, SiN2 and Si(CO)2 molecules: electron spin resonance and optical spectra at 4 K. J. Am. Chem. Soc. 99, 416–423 (1977).
Grev, R. S. & Schaefer, H. F. III Reassignment of the structure of Si(CO)2 based on theoretically predicted IR spectra. J. Am. Chem. Soc. 111, 5691–5699 (1989).
Zhou, M., Jiang, L. & Xu, Q. Reactions of silicon atoms and small clusters with CO: experimental and theoretical characterization of Si nCO (n = 1–5),Si2(CO)2, c-Si2(μ-O)(μ-CSi) and c-Si2(μ-O)(μ-CCO) in solid argon. J. Chem. Phys. 121, 10474–10482 (2004).
Goedecke, C., Leibold, M., Siemeling, U. & Frenking, G. When does carbonylation of carbenes yield ketenes? A theoretical study with implications for synthesis. J. Am. Chem. Soc. 133, 3557–3569 (2011).
Becerra, R. & Walsh, R. Silylene does react with carbon monoxide. J. Am. Chem. Soc. 122, 3246–3247 (2000).
Becerra, R., Cannady, J. P. & Walsh, R. Silylene does react with carbon monoxide: some gas-phase kinetic and theoretical studies. J. Phys. Chem. A 105, 1897–1903 (2001).
Chu, J. H., Beach, D. B., Estes, R. D. & Jasinski, J. M. Absolute rate constants for silylene reactions with diatomic molecules. Chem. Phys. Lett. 143, 135–139 (1988).
Maier, G., Reisenauer, H.-P. & Egenolf, H. Quest for silaketene: a matrix-spectroscopic and theoretical study. Organometallics 18, 2155–2161 (1999).
Pearsall, M. A. & West, R. The reactions of diorganosilylenes with carbon monoxide. J. Am. Chem. Soc. 110, 7228–7229 (1988).
Arrington, C. A., Petty, J. T., Payne, S. E. & Haskins, W. C. K. The reaction of dimethylsilylene with carbon monoxide in low-temperature matrices. J. Am. Chem. Soc. 110, 6240–6241 (1988).
Tacke, M. et al. Complexes of decamethylsilicocene: Cp2*Si(CO) and Cp2*Si(N2). Z. Anorg. Allg. Chem. 619, 865–868 (1993).
Bornemann, H. & Sander, W. Reactions of methyl(phenyl)silylene with CO and PH3—the formation of acid–base complexes. J. Organomet. Chem. 641, 156–164 (2002).
Wang, Y. et al. Silicon-mediated selective homo- and heterocoupling of carbon monoxide. J. Am. Chem. Soc. 141, 626–634 (2019).
Protchenko, A. V. et al. Reduction of carbon oxides by an acyclic silylene: reductive coupling of CO. Angew. Chem. Int. Ed. 58, 1808–1812 (2019).
Protchenko, A. V. et al. A stable two-coordinate acyclic silylene. J. Am. Chem. Soc. 134, 6500–6503 (2012).
Wang, X. et al. Room-temperature reaction of carbon monoxide with a stable diarylgermylene. J. Am. Chem. Soc. 131, 6912–6913 (2009).
Brown, Z. D. & Power, P. P. Mechanisms of reactions of open-shell, heavier group 14 derivatives with small molecules: n–π* back-bonding in isocyanide complexes, C–H activation under ambient conditions, CO coupling and ancillary molecular interactions. Inorg. Chem. 52, 6248–6259 (2013).
Chu, T. & Nikonov, G. I. Oxidative addition and reductive elimination at main-group element centers. Chem. Rev. 118, 3608–3680 (2018).
Krüger, J., Ganesamoorthy, C., John, L., Wölper, C. & Schulz, S. A general pathway for the synthesis of gallastibenes containing Ga=Sb double bonds. Chem. Eur. J. 24, 9157–9164 (2018).
Helling, C., Wölper, C. & Schulz, S. Synthesis of a gallaarsene {HC[C(Me)N-2,6-i-Pr2-C6H3]2}GaAsCp* containing a Ga=As double bond. J. Am. Chem. Soc. 140, 5053–5056 (2018).
Ganesamoorthy, C. et al. From stable Sb- and Bi-centered radicals to a compound with a Ga=Sb double bond. Nat. Commun. 9, 87 (2018).
Zhu, L., Zhang, J. & Cui, C. Intramolecular cyclopropanation of alkali-metal-substituted silylene with the aryl substituent of an N-heterocyclic framework. Inorg. Chem. 58, 12007–12010 (2019).
Gau, D. et al. Synthesis of a stable disilyne bisphosphine adduct and its non-metal-mediated CO2 reduction to CO. Angew. Chem. Int. Ed. 50, 1092–1096 (2011).
Takeda, N., Suzuki, H., Tokitoh, N. & Okazaki, R. Reaction of a sterically hindered silylene with isocyanides: the first stable silylene–Lewis base complexes. J. Am. Chem. Soc. 119, 1456–1457 (1997).
Takeda, N., Kajiwara, T., Suzuki, H., R. Okazaki, R. & Tokitoh, N. Synthesis and properties of the first stable silylene isocyanide complexes. Chem. Eur. J. 9, 3530–3543 (2003).
Kempter, A., Gemel, C. & Fischer, R. A. Oxidative addition of group 13 and 14 metal halides and alkyls to Ga(DDP) (DDP = bulky bisimidinate). Inorg. Chem. 47, 7279–7285 (2008).
Huber, K. P. & Herzberg. G. Molecular Spectra and Molecular Structure IV: Constants of Diatomic Molecules 166 (Van Nostrand Reinhold Co, 1979).
Frisch, M. J. et al. Gaussian 16, Revision C.01 (Gaussian, 2016).
Müller, T. The chemical shift tensor of silylenes. J. Organomet. Chem. 686, 251–256 (2003).
Holthausen, M. C., Koch, W. & Apeloig, Y. Theory predicts triplet ground-state organic silylenes. J. Am. Chem. Soc. 121, 2623–2624 (1999).
Blom, B. & Driess, M. in Structure and Bonding Vol. 156 (ed. Scheschkewitz, D.) 85–124 (Springer, 2014).
Acknowledgements
This work was supported by the University of Duisburg–Essen and the Alexander-von-Humboldt Foundation (a scholarship to L.S.). We thank the Deutsche Forschungsgemeinschaft for partial support within the Priority Program SPP 1807 (control of London dispersion interactions in molecular chemistry) and T. Benter and H. Kersten for mass spectroscopy measurements.
Author information
Authors and Affiliations
Contributions
C.G., S.S. and P.R.S. conceived the experiments. S.S. and P.R.S. supervised the study. C.G. and J.S. performed the synthetic studies, C.W. the single-crystal X-ray diffraction studies, and L.S. the computational experiments. C.G., S.S. and P.R.S. wrote the manuscript, with input from all authors. All authors analysed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Experimental details including methods, synthetic procedures, characterization data (NMR, IR, UV–vis spectra), crystallographic details and computational details.
Crystallographic data
CIF for compound 1; CCDC reference 1943186
Crystallographic data
CIF for compound 2; CCDC reference 1943187
Crystallographic data
CIF for compound 3; CCDC reference 1943188
Crystallographic data
CIF for compound 4; CCDC reference 1943189
Crystallographic data
CIF for compound 5; CCDC reference 1943190
Crystallographic data
CIF for compound 6; CCDC reference 1967024
Rights and permissions
About this article
Cite this article
Ganesamoorthy, C., Schoening, J., Wölper, C. et al. A silicon–carbonyl complex stable at room temperature. Nat. Chem. 12, 608–614 (2020). https://doi.org/10.1038/s41557-020-0456-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0456-x
This article is cited by
-
A class of non-aromatic 1,3-disilapyrroles acting as stable organosilicon-based triplet diradicals
Nature Synthesis (2023)
-
Synthesis, isolation and application of a sila-ketenyl anion
Nature Synthesis (2023)
-
Illuminating the dark conformational space of macrocycles using dominant rotors
Nature Chemistry (2021)
-
Nonclassical complex of dichlorosilylene with CO: direct spectroscopic detection
Russian Chemical Bulletin (2021)
-
Surprisingly stable Si–CO species
Nature Chemistry (2020)