Abstract
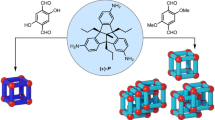
When an amide group is distorted from its planar conformation, the conjugation between the nitrogen lone pair and the π* orbital of the carbonyl is disrupted and the reactivity towards nucleophiles is enhanced. Although there are several reports on the synthesis of activated twisted amides, amide activation through mechanical twisting is much less common. Here, we report twisted amides that are stabilized through their inclusion in a self-assembled coordination cage. When secondary aromatic amides are included in a Td-symmetric cage, the cis-twisted conformation is favoured over the trans-planar one—as evidenced by single-crystal X-ray diffraction analysis—revealing that the amide can twist by up to 34°. As a consequence of this distortion, the hydrolysis of amides is significantly accelerated upon inclusion.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within this article and its Supplementary Information. Crystallographic data for inclusion complexes 1b•(2a)2, 1a•(2b)2, 1a•(2b•3a), 1a•(2b•4), 1a•(2c)2, 1a•2d and 1a•(2e)2 are free of charge from the Cambridge Crystallographic Data Centre (www.ccdc.cam.ac.uk) under reference nos. 1949143, 1949144, 1949145, 1949146, 1949149, 1949150 and 1949151, respectively.
References
Alabugin, I. V. Stereoelectronic Effects: A Bridge Between Structure and Reactivity (Wiley, 2016).
Greenberg, A., Breneman, C. M. & Liebman, J. F. The Amide Linkage: Selected Structural Aspects in Chemistry, Biochemistry and Materials Science (Wiley, 2000).
Yamada, S. Structure and reactivity of a highly twisted amide. Angew. Chem. Int. Ed. 32, 1083–1085 (1993).
Kirby, A. J., Komarov, I. V. & Feeder, N. Synthesis, structure and reactions of the most twisted amide. J. Chem. Soc. Perkin Trans. 2, 522–529 (2001).
Tani, K. & Stoltz, B. M. Synthesis and structural analysis of 2-quinuclidonium tetrafluoroborate. Nature 441, 731–734 (2006).
Hutchby, M. et al. Switching pathways: room-temperature neutral solvolysis and substitution of amides. Angew. Chem. Int. Ed. 51, 548–551 (2012).
Szostak, M. & Aubé, J. Chemistry of bridged lactams and related heterocycles. Chem. Rev. 113, 5701–5765 (2013).
Komarov, I. V. et al. The most reactive amide as a transition-state mimic for cis–trans interconversion. J. Am. Chem. Soc. 137, 926–930 (2015).
Liniger, M., VanderVelde, D. G., Takase, M. K., Shahgholi, M. & Stoltz, B. M. Total synthesis and characterization of 7-hypoquinuclidonium tetrafluoroborate and 7-hypoquinuclidone BF3 complex. J. Am. Chem. Soc. 138, 969–974 (2016).
Liu, C. & Szostak, M. Twisted amides: from obscurity to broadly useful transition-metal-catalyzed reactions by N–C amide bond activation. Chem. Eur. J. 23, 7157–7173 (2017).
Meng, G., Shi, S., Lalancette, R., Szostak, R. & Szostak, M. Reversible twisting of primary amides via ground state N–C(O) destabilization: highly twisted rotationally inverted acyclic amides. J. Am. Chem. Soc. 140, 727–734 (2018).
Adachi, S., Kumagai, N. & Shibasaki, M. Pyramidalization/twisting of the amide functional group via remote steric congestion triggered by metal coordination. Chem. Sci. 8, 85–90 (2017).
Romanelli, A., Shekhtman, A., Cowburn, D. & Muir, T. W. Semisynthesis of a segmental isotopically labeled protein splicing precursor: NMR evidence for an unusual peptide bond at the N-extein–intein junction. Proc. Natl Acad. Sci. USA 101, 6397–6402 (2004).
Macao, B., Johansson, D. G, Hansson, G. C. & Härd, T. Autoproteolysis coupled to protein folding in the SEA domain of the membrane-bound MUC1 mucin. Nat. Struct. Mol. Biol. 13, 71–76 (2006).
Johansson, D. G. A., Macao, B., Sandberg, A. & Härd, T. SEA domain autoproteolysis accelerated by conformational strain: mechanistic aspects. J. Mol. Biol. 377, 1130–1143 (2008).
Johansson, D. G. A. et al. Protein autoproteolysis: conformational strain linked to the rate of peptide cleavage by the pH dependence of the N → O acyl shift reaction. J. Am. Chem. Soc. 131, 9475–9477 (2009).
Wallin, G., Härd, T. & Åqvist, J. Folding-reaction coupling in a self-cleaving protein. J. Chem. Theory Comput. 8, 3871–3879 (2012).
Lizak, C. et al. Unexpected reactivity and mechanism of carboxamide activation in bacterial N-linked protein glycosylation. Nat. Commun. 4, 2627 (2013).
Pernía, G. J. et al. Stabilization of a cis amide bond in a host–guest complex. J. Am. Chem. Soc. 118, 10220–10227 (1996).
Pluth, M. D., Bergman, R. G. & Raymond, K. N. Acceleration of amide bond rotation by encapsulation in the hydrophobic interior of a water-soluble supramolecular assembly. J. Org. Chem. 73, 7132–7136 (2008).
Escobar, L., Díaz-Moscoso, A. & Ballester, P. Conformational selectivity and high-affinity binding in the complexation of N-phenyl amides in water by a phenyl extended calix[4]pyrrole. Chem. Sci. 9, 7186–7192 (2018).
Fujita, M. et al. Self-assembly of ten molecules into nanometre-sized organic host frameworks. Nature 378, 469–471 (1995).
Kusukawa, T., Yoshizawa, M. & Fujita, M. Probing guest geometry and dynamics through host–guest interactions. Angew. Chem. Int. Ed. 40, 1879–1884 (2001).
Yoshizawa, M., Sato, N. & Fujita, M. Selective enclathration of linear alkanols by a self-assembled coordination cage. Application to the catalytic Wacker oxidation of ω-alkenols. Chem. Lett. 34, 1392–1393 (2005).
Takezawa, H., Murase, T., Resnati, G., Metrangolo, P. & Fujita, M. Recognition of polyfluorinated compounds through self-aggregation in a cavity. J. Am. Chem. Soc. 136, 1786–1788 (2014).
Takezawa, H., Murase, T. & Fujita, M. Temporary and permanent trapping of the metastable twisted conformer of an overcrowded chromic alkene via encapsulation. J. Am. Chem. Soc. 134, 17420–17423 (2012).
Takezawa, H., Akiba, S., Murase, T. & Fujita, M. Cavity-directed chromism of phthalein dyes. J. Am. Chem. Soc. 137, 7043–7046 (2015).
Ibukuro, F., Kusukawa, T. & Fujita, M. A thermally switchable molecular lock. Guest-templated synthesis of a kinetically stable nanosized cage. J. Am. Chem. Soc. 120, 8561–8562 (1998).
Yoshizawa, M., Tamura, M. & Fujita, M. AND/OR bimolecular recognition. J. Am. Chem. Soc. 126, 6846–6847 (2004).
Acknowledgements
This research was supported by Grants-in-Aid for Specially Promoted Research (19H05461 to M.F.), for Young Scientists (19K15581 to H.T.) and the Noguchi Institute (to H.T.).
Author information
Authors and Affiliations
Contributions
H.T. and K.S. performed the described experiments and analysed the data. H.T. and M.F. conceived and designed the experiments and co-wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary experimental details and compound characterization data.
Crystallographic data
CIF for 1b·(2a)2; CCDC reference: 1949143.
Crystallographic data
CIF for 1a·(2b)2; CCDC reference: 1949144.
Crystallographic data
CIF for 1a·(2b·3a); CCDC reference: 1949145.
Crystallographic data
CIF for 1a·(2b·4); CCDC reference: 1949146.
Crystallographic data
CIF for 1a·(2c)2; CCDC reference: 1949149.
Crystallographic data
CIF for 1a·2d; CCDC reference: 1949150.
Crystallographic data
CIF for 1a·(2e)2·S1; CCDC reference: 1949151.
Rights and permissions
About this article
Cite this article
Takezawa, H., Shitozawa, K. & Fujita, M. Enhanced reactivity of twisted amides inside a molecular cage. Nat. Chem. 12, 574–578 (2020). https://doi.org/10.1038/s41557-020-0455-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-0455-y
This article is cited by
-
Photoswitchable coordination cages
Nature Chemistry (2024)
-
Chiral BINOL-phosphate assembled single hexagonal nanotube in aqueous solution for confined rearrangement acceleration
Nature Communications (2024)
-
Composite Nanoarchitectonics Towards Method for Everything in Materials Science
Journal of Inorganic and Organometallic Polymers and Materials (2024)
-
Kinetic trapping of 2,4,6-tris(4-pyridyl)benzene and ZnI2 into M12L8 poly-[n]-catenanes using solution and solid-state processes
Scientific Reports (2023)
-
Metal-organic cages containing two types of binding sites: trapping hydrocarbon gas in solution
Science China Chemistry (2023)