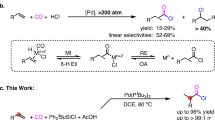
Abstract
Hydroformylation, a reaction that installs both a C–H bond and an aldehyde group across an unsaturated substrate, is one of the most important catalytic reactions in both industry and academia. Given the synthetic importance of creating new C–C bonds, the development of carboformylation reactions, wherein a new C–C bond is formed instead of a C–H bond, would bear enormous synthetic potential to rapidly increase molecular complexity in the synthesis of valuable aldehydes. However, the demanding complexity inherent in a four-component reaction, utilizing an exogenous CO source, has made the development of a direct carboformylation reaction a formidable challenge. Here, we describe a palladium-catalysed strategy that uses readily available aroyl chlorides as a carbon electrophile and CO source, in tandem with a sterically congested hydrosilane, to perform a stereoselective carboformylation of alkynes. An extension of this protocol to four chemodivergent carbonylations further highlights the creative opportunity offered by this strategy in carbonylation chemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1998285 (10), 2018417 (33), 2000217 (36, minor), 2000218 (53), 2018418 (59), 2018419 (59-Z), 1998290 (62), 1998292 (64), 1998293 (L08) and 1998299 (76). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data is available in the main text or the Supplementary Information.
References
Bertleff, W., Roeper, M. & Sava, X. in Ullmann’s Encyclopedia of Industrial Chemistry 73–95 (Wiley, 2007).
Börner, A. & Franke, R. Hydroformylation: Fundamentals, Processes and Applications in Organic Synthesis (Wiley, 2016).
Cornils, B., Herrmann, W. A. & Rasch, M. Otto Roelen, pioneer in industrial homogeneous catalysis. Angew. Chem. Int. Ed. 33, 2144–2163 (1994).
Kiss, G. Palladium-catalyzed Reppe carbonylation. Chem. Rev. 101, 3435–3456 (2001).
Peng, J.-B., Wu, F.-P. & Wu, X.-F. First-row transition-metal-catalyzed carbonylative transformations of carbon electrophiles. Chem. Rev. 119, 2090–2127 (2019).
Bai, Y., Davis, D. C. & Dai, M. Natural product synthesis via palladium-catalyzed carbonylation. J. Org. Chem. 82, 2319–2328 (2017).
Hood, D. M. et al. Highly active cationic cobalt(ii) hydroformylation catalysts. Science 367, 542–548 (2020).
Yang, J. et al. Direct synthesis of adipic acid esters via palladium-catalyzed carbonylation of 1,3-dienes. Science 366, 1514–1517 (2019).
Willcox, D. et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science 354, 851–857 (2016).
Kinney, R. G. et al. A general approach to intermolecular carbonylation of arene C–H bonds to ketones through catalytic aroyl triflate formation. Nat. Chem. 10, 193–199 (2018).
Schoenberg, A. & Heck, R. F. Palladium-catalyzed formylation of aryl, heterocyclic and vinylic halides. J. Am. Chem. Soc. 96, 7761–7764 (1974).
Baillargeon, V. P. & Stille, J. K. Palladium-catalyzed formylation of organic halides with carbon monoxide and tin hydride. J. Am. Chem. Soc. 108, 452–461 (1986).
Pri-Bar, I. & Buchman, O. Reductive formylation of aromatic halides under low carbon monoxide pressure catalyzed by transition-metal compounds. J. Org. Chem. 49, 4009–4011 (1984).
Wu, X.-F. et al. Development of a general palladium-catalyzed carbonylative Heck reaction of aryl halides. J. Am. Chem. Soc. 132, 14596–14602 (2010).
Wu, L., Liu, Q., Jackstell, R. & Beller, M. Carbonylations of alkenes with CO surrogates. Angew. Chem. Int. Ed. 53, 6310–6320 (2014).
Morimoto, T. & Kakiuchi, K. Evolution of carbonylation catalysis: no need for carbon monoxide. Angew. Chem. Int. Ed. 43, 5580–5588 (2004).
Friis, S. D., Lindhardt, A. T. & Skrydstrup, T. The development and application of two-chamber reactors and carbon monoxide precursors for safe carbonylation reactions. Acc. Chem. Res. 49, 594–605 (2016).
Murphy, S. K., Park, J.-W., Cruz, F. A. & Dong, V. M. Rh-catalyzed C–C bond cleavage by transfer hydroformylation. Science 347, 56–60 (2015).
Tan, G., Wu, Y., Shi, Y. & You, J. Syngas-free rhodium-catalyzed highly regioselective transfer hydroformylation of alkynes to α,β-unsaturated aldehydes. Angew. Chem. Int. Ed. 58, 7440–7444 (2019).
Fang, X., Cacherat, B. & Morandi, B. CO- and HCl-free synthesis of acid chlorides from unsaturated hydrocarbons via shuttle catalysis. Nat. Chem. 9, 1105–1109 (2017).
Lee, Y. H. & Morandi, B. Metathesis-active ligands enable a catalytic functional group metathesis between aroyl chlorides and aryl iodides. Nat. Chem. 10, 1016–1022 (2018).
De La Higuera Macias, M. & Arndtsen, B. A. Functional group transposition: a palladium-catalyzed metathesis of Ar–X σ-bonds and acid chloride synthesis. J. Am. Chem. Soc. 140, 10140–10144 (2018).
Johnson, J. R., Cuny, G. D. & Buchwald, S. L. Rhodium‐catalyzed hydroformylation of internal alkynes to α,β‐unsaturated aldehydes. Angew. Chem. Int. Ed. 34, 1760–1761 (1995).
Zhang, S., Neumann, H. & Beller, M. Synthesis of α,β-unsaturated carbonyl compounds by carbonylation reactions. Chem. Soc. Rev. 49, 3187–3210 (2020).
Matsuda, I., Ogiso, A., Sato, S. & Izumi, Y. An efficient silylformylation of alkynes catalyzed by tetrarhodium dodecacarbonyl. J. Am. Chem. Soc. 111, 2332–2333 (1989).
Marek, I., Chinkov, N. & Banon-Tenne, D. in Metal-Catalyzed Cross-Coupling Reactions (eds de Meijere, A. & Diederich, F.) 395–478 (Wiley, 2004).
Brown, S., Clarkson, S., Grigg, R. & Sridharan, V. Palladium-catalysed cyclisation-carboformylation. Molecular queuing cascades. J. Chem. Soc. Chem. Commun. 1995, 1135–1136 (1995).
Brown, S. et al. Palladium catalysed queuing processes. Part 1: termolecular cyclization-anion capture employing carbon monoxide as a relay switch and hydride, organotin(iv) or boron reagents. Tetrahedron 57, 1347–1359 (2001).
Negishi, E. & Copéret, C. in Handbook of Organopalladium Chemistry for Organic Synthesis (ed. Negishi, E.) 1431–1448 (Wiley, 2002).
Van den Hoven, B. G. & Alper, H. Innovative synthesis of 4-carbaldehydepyrrolin-2-ones by zwitterionic rhodium catalyzed chemo- and regioselective tandem cyclohydrocarbonylation/CO insertion of α-imino alkynes. J. Am. Chem. Soc. 123, 10214–10220 (2001).
Fuji, K., Morimoto, T., Tsutsumi, K. & Kakiuchi, K. Rh(i)-catalyzed CO gas-free cyclohydrocarbonylation of alkynes with formaldehyde to α,β-butenolides. Chem. Commun. 3295–3297 (2005); https://doi.org/10.1039/b503816b
Peng, J.-B., Wu, F.-P., Spannenberg, A. & Wu, X.-F. Palladium-catalyzed tunable carbonylative synthesis of enones and benzofulvenes. Chem. Eur. J. 25, 8696–8700 (2019).
Fujihara, T., Tatsumi, K., Terao, J. & Tsuji, Y. Palladium-catalyzed formal hydroacylation of allenes employing acid chlorides and hydrosilanes. Org. Lett. 15, 2286–2289 (2013).
Kokubo, K., Matsumasa, K., Miura, M. & Nomura, M. Rhodium-catalyzed reaction of aroyl chlorides with alkynes. J. Org. Chem. 61, 6941–6946 (1996).
Yasukawa, T., Satoh, T., Miura, M. & Nomura, M. Iridium-catalyzed reaction of aroyl chlorides with internal alkynes to produce substituted naphthalenes and anthracenes. J. Am. Chem. Soc. 124, 12680–12681 (2002).
Wang, P., Rao, H., Zhou, F., Hua, R. & Li, C.-J. Ruthenium-catalyzed aldehyde functionality reshuffle: selective synthesis of E‑2-arylcinnamaldehydes from E-β-bromostyrenes and aryl aldehydes. J. Am. Chem. Soc. 134, 16468–16471 (2012).
Chen, P., Billett, B. A., Tsukamoto, T. & Dong, G. ‘Cut and sew’ transformations via transition-metal-catalyzed carbon−carbon bond activation. ACS Catal. 7, 1340–1360 (2017).
Tsuji, J. in Handbook of Organopalladium Chemistry for Organic Synthesis (ed. Negishi, E.) 2643–2653 (Wiley, 2002).
Shen, X., Hyde, A. M. & Buchwald, S. L. Palladium-catalyzed conversion of aryl and vinyl triflates to bromides and chlorides. J. Am. Chem. Soc. 132, 14076–14078 (2010).
Petrone, D. A., Ye, J. & Lautens, M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem. Rev. 116, 8003–8104 (2016).
Malapit, C. A., Ichiishi, N. & Sanford, M. S. Pd-catalyzed decarbonylative cross-couplings of aroyl chlorides. Org. Lett. 19, 4142–4145 (2017).
Flynn, A. B. & Ogilvie, W. W. Stereocontrolled synthesis of tetrasubstituted olefins. Chem. Rev. 107, 4698–4745 (2007).
Casey, C. P. et al. Diphosphines with natural bite angles near 120° increase selectivity for n-aldehyde formation in rhodium-catalyzed hydroformylation. J. Am. Chem. Soc. 114, 5535–5543 (1992).
Trost, B. M., Sorum, M. T., Chan, C., Harms, A. E. & Rühter, G. Palladium-catalyzed additions of terminal alkynes to acceptor alkynes. J. Am. Chem. Soc. 119, 698–708 (1997).
El-Khawaga, A. M., Roberts, R. M. & Sweeney, K. M. Transacylations between sterically hindered aromatic ketones and various arenes. J. Org. Chem. 50, 2055–2058 (1985).
Gauthier, D. R. Jr, Rivera, N. R., Yang, H., Schultz, D. M. & Shultz, C. S. Palladium-catalyzed carbon isotope exchange on aliphatic and benzoic acid chlorides. J. Am. Chem. Soc. 140, 15596–15600 (2018).
Natta, G., Ercoli, R., Castellano, S. & Barbieri, F. H. The influence of hydrogen and carbon monoxide partial pressures on the rate of the hydroformylation reaction. J. Am. Chem. Soc. 76, 4049–4050 (1954).
Cnossen, A., Browne, W. R. & Feringa, B. L. in Molecular Machines and Motors (eds Credi, A. et al.) 139–162 (Springer, 2014).
Meier, H. The photochemistry of stilbenoid compounds and their role in materials technology. Angew. Chem. Int. Ed. 31, 1399–1420 (1992).
Mei, J., Leung, N. L. C., Kwok, R. T. K., Lam, J. W. Y. & Tang, B. Z. Aggregation-induced emission: together we shine, united we soar! Chem. Rev. 115, 11718–11940 (2015).
Chan, J., Dodani, S. C. & Chang, C. J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 4, 973–984 (2012).
Acknowledgements
We acknowledge ETH Zurich, the European Research Council under the European Union’s Horizon 2020 research and innovation programme (Shuttle Cat, project ID 757608) and LG Chem (fellowship to Y.H.L.) for financial support. We thank the NMR, MS (MoBiAS) and X-ray (SMoCC) service departments at ETH Zurich for technical assistance.
Author information
Authors and Affiliations
Contributions
Y.H.L. designed and discovered the reaction. Y.H.L. and E.H.D. deterimined the scope of the reaction. B.M. supervised the research. All authors contributed to manuscript writing and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Tables 1–28, equations 1–40, experimental details for all reactions and analytic details for all products.
Supplementary Data 1
Crystallographic data for compound 10. CCDC reference 1998285.
Supplementary Data 2
Crystallographic data for compound 33. CCDC reference 2018417.
Supplementary Data 3
Crystallographic data for compound 36-alpha-Ar, minor. CCDC reference 2000217.
Supplementary Data 4
Crystallographic data for compound 53. CCDC reference 2000218.
Supplementary Data 5
Crystallographic data for compound 59. CCDC reference 2018418.
Supplementary Data 6
Crystallographic data for compound 59-Z. CCDC reference 2018419.
Supplementary Data 7
Crystallographic data for compound 62. CCDC reference 1998290.
Supplementary Data 8
Crystallographic data for compound 64. CCDC reference 1998292.
Supplementary Data 9
Crystallographic data for compound L08. CCDC reference 1998293.
Supplementary Data 10
Crystallographic data for compound 76. CCDC reference 1998299.
Rights and permissions
About this article
Cite this article
Lee, Y.H., Denton, E.H. & Morandi, B. Palladium-catalysed carboformylation of alkynes using acid chlorides as a dual carbon monoxide and carbon source. Nat. Chem. 13, 123–130 (2021). https://doi.org/10.1038/s41557-020-00621-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00621-x
This article is cited by
-
Fragmentation and reassembly
Nature Chemistry (2021)