Abstract
Membrane proteins on the cell surface perform a myriad of biological functions; however, ligand discovery for membrane proteins is highly challenging, because a natural cellular environment is often necessary to maintain protein structure and function. DNA-encoded chemical libraries (DELs) have emerged as a powerful technology for ligand discovery, but they are mainly limited to purified proteins. Here we report a method that can specifically label membrane proteins with a DNA tag, and thereby enable target-specific DEL selections against endogenous membrane proteins on live cells without overexpression or any other genetic manipulation. We demonstrate the generality and performance of this method by screening a 30.42-million-compound DEL against the folate receptor, carbonic anhydrase 12 and the epidermal growth factor receptor on live cells, and identify and validate a series of novel ligands for these targets. Given the high therapeutic significance of membrane proteins and their intractability to traditional high-throughput screening approaches, this method has the potential to facilitate membrane-protein-based drug discovery by harnessing the power of DEL.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article, the associated Source Data files, Supplementary Information and Extended Data files. All the published tools and packages used for data analysis are provided with the paper. The Human UniProt database (release-2016_05) used can be accessed at https://www.uniprot.org/proteomes/UP000005640, and the BioNumbers database can be accessed at https://bionumbers.hms.harvard.edu/. Source data are provided with this paper.
Code availability
The custom Python script for sequencing data analysis is freely available for downloading both as part of Supplementary Information and also at GitHub (https://github.com/cenhuang0916/sequencing-data-processing-script.git)
References
Cournia, Z. et al. Membrane protein structure, function, and dynamics: a perspective from experiments and theory. J. Membr. Biol. 248, 611–640 (2015).
Yin, H. & Flynn, A. D. Drugging membrane protein interactions. Annu. Rev. Biomed. Eng. 18, 51–76 (2016).
Hauser, A. S., Attwood, M. M., Rask-Andersen, M., Schioth, H. B. & Gloriam, D. E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug. Discov. 16, 829–842 (2017).
Li, X. L., Shao, C. S., Shi, Y. F. & Han, W. D. Lessons learned from the blockade of immune checkpoints in cancer immunotherapy. J. Hematol. Oncol. 11, 31 (2018).
Rawlings, A. E. Membrane proteins: always an insoluble problem? Biochem. Soc. Trans. 44, 790–795 (2016).
Brenner, S. & Lerner, R. A. Encoded combinatorial chemistry. Proc. Natl Acad. Sci. USA 89, 5381–5383 (1992).
Nielsen, J., Brenner, S. & Janda, K. D. Synthetic methods for the implementation of encoded combinatorial chemistry. J. Am. Chem. Soc. 115, 9812–9813 (1993).
Neri, D. & Lerner, R. A. DNA-encoded chemical libraries: a selection system based on endowing organic compounds with amplifiable information. Annu. Rev. Biochem. 87, 479–502 (2018).
Zhao, G., Huang, Y., Zhou, Y., Li, Y. & Li, X. Future challenges with DNA-encoded chemical libraries in the drug discovery domain. Expert Opin. Drug Discov. 14, 735–753 (2019).
Ottl, J., Leder, L., Schaefer, J. V. & Dumelin, C. E. Encoded library technologies as integrated lead finding platforms for drug discovery. Molecules 24, 1629 (2019).
Dickson, P. & Kodadek, T. Chemical composition of DNA-encoded libraries, past present and future. Org. Biomol. Chem. 17, 4676–4688 (2019).
Kodadek, T., Paciaroni, N. G., Balzarini, M. & Dickson, P. Beyond protein binding: recent advances in screening DNA-encoded libraries. Chem. Commun. 55, 13330–13341 (2019).
Huang, Y., Savych, O., Moroz, Y., Chen, Y. Y. & Goodnow, R. A. DNA-encoded library chemistry: amplification of chemical reaction diversity for the exploration of chemical space. Aldrichim. Acta 52, 75–87 (2019).
Song, M. & Hwang, G. T. DNA-encoded library screening as a core platform technology in drug discovery: its synthetic method development and applications in DEL synthesis. J. Med. Chem. 63, 6578–6599 (2020).
Halford, B. Breakthroughs with bar codes—DNA-encoded libraries help pharma find drug leads. Chem. Eng. News 95, 28–33 (2017).
Arico-Muendel, C. C. From haystack to needle: finding value with DNA encoded library technology at GSK. MedChemComm 7, 1898–1909 (2016).
Goodnow, R. A. A Handbook for DNA-Encoded Chemistry: Theory and Applications for Exploring Chemical Space and Drug Discovery (John Wiley & Sons, 2014).
Buller, F. et al. Selection of carbonic anhydrase IX Inhibitors from one million DNA-encoded compounds. ACS Chem. Biol. 6, 336–344 (2011).
Kollmann, C. S. et al. Application of encoded library technology (ELT) to a protein–protein interaction target: discovery of a potent class of integrin lymphocyte function-associated antigen 1 (LFA-1) antagonists. Bioorg. Med. Chem. 22, 2353–2365 (2014).
Wichert, M. et al. Dual-display of small molecules enables the discovery of ligand pairs and facilitates affinity maturation. Nat. Chem. 7, 241–249 (2015).
Leimbacher, M. et al. Discovery of small-molecule interleukin-2 inhibitors from a DNA-encoded chemical library. Chem. Eur. J. 18, 7729–7737 (2012).
Richter, H. et al. DNA-encoded library-derived DDR1 inhibitor prevents fibrosis and renal function loss in a genetic mouse model of Alport syndrome. ACS Chem. Biol. 14, 37–49 (2019).
Ahn, S. et al. Allosteric ‘beta-blocker’ isolated from a DNA-encoded small molecule library. Proc. Natl Acad. Sci. USA 114, 1708–1713 (2017).
Ahn, S. et al. Small-molecule positive allosteric modulators of the β2-adrenoceptor isolated from DNA-encoded libraries. Mol. Pharmacol. 94, 850–861 (2018).
Zhao, P. et al. Selection of DNA-encoded small molecule libraries against unmodified and non-immobilized protein targets. Angew. Chem. Int. Ed. 53, 10056–10059 (2014).
Shi, B., Deng, Y., Zhao, P. & Li, X. Selecting a DNA-encoded chemical library against non-immobilized proteins using a ‘ligate–cross-link–purify” strategy. Bioconjugate Chem. 28, 2293–2301 (2017).
Blakskjaer P. et al. A method for making an enriched library patent. WO patent 2012041633 A1, 2012.4.5. (2012).
Bao, J. et al. Predicting electrophoretic mobility of protein–ligand complexes for ligands from DNA-encoded libraries of small molecules. Anal. Chem. 88, 5498–5506 (2016).
McGregor, L. M., Jain, T. & Liu, D. R. Identification of ligand–target pairs from combined libraries of small molecules and unpurified protein targets in cell lysates. J. Am. Chem. Soc. 136, 3264–3270 (2014).
Denton, K. E. & Krusemark, C. J. Crosslinking of DNA-linked ligands to target proteins for enrichment from DNA-encoded libraries. MedChemComm 7, 2020–2027 (2016).
Kim, D. et al. Application of a substrate-mediated selection with c-Src tyrosine kinase to a DNA-encoded chemical library. Molecules 24, 2764 (2019).
Zhou, Y. et al. DNA-encoded dynamic chemical library and its applications in ligand discovery. J. Am. Chem. Soc. 140, 15859–15867 (2018).
Wu, Z. et al. Cell-based selection expands the utility of DNA-encoded small-molecule library technology to cell surface drug targets: identification of novel antagonists of the NK3 tachykinin receptor. ACS Comb. Sci. 17, 722–731 (2015).
Svensen, N., Diaz-Mochon, J. J. & Bradley, M. Decoding a PNA encoded peptide library by PCR: the discovery of new cell surface receptor ligands. Chem. Biol. 18, 1284–1289 (2011).
Svensen, N., Diaz-Mochon, J. J. & Bradley, M. Encoded peptide libraries and the discovery of new cell binding ligands. Chem. Commun. 47, 7638–7640 (2011).
Yan, M. et al. Next-generation glycan microarray enabled by DNA-coded glycan library and next-generation sequencing technology. Anal. Chem. 91, 9221–9228 (2019).
Cai, B. et al. Selection of DNA-encoded libraries to protein targets within and on living cells. J. Am. Chem. Soc. 141, 17057–17061 (2019).
Decurtins, W. et al. Automated screening for small organic ligands using DNA-encoded chemical libraries. Nat. Protoc. 11, 764–780 (2016).
Chandra, R. A., Douglas, E. S., Mathies, R. A., Bertozzi, C. R. & Francis, M. B. Programmable cell adhesion encoded by DNA hybridization. Angew. Chem. Int. Ed. 45, 896–901 (2006).
Gartner, Z. J. & Bertozzi, C. R. Programmed assembly of 3-dimensional microtissues with defined cellular connectivity. Proc. Natl Acad. Sci. USA 106, 4606–4610 (2009).
Furst, A. L., Smith, M. J. & Francis, M. B. Direct electrochemical bioconjugation on metal surfaces. J. Am. Chem. Soc. 139, 12610–12616 (2017).
Zhao, W. et al. Mimicking the inflammatory cell adhesion cascade by nucleic acid aptamer programmed cell–cell interactions. FASEB J. 25, 3045–3056 (2011).
El Muslemany, K. M. et al. Photoactivated bioconjugation between ortho-azidophenols and anilines: a facile approach to biomolecular photopatterning. J. Am. Chem. Soc. 136, 12600–12606 (2014).
Vogel, K., Glettenberg, M., Schroeder, H. & Niemeyer, C. M. DNA-modification of eukaryotic cells. Small 9, 255–262 (2013).
Meyer, R., Giselbrecht, S., Rapp, B. E., Hirtz, M. & Niemeyer, C. M. Advances in DNA-directed immobilization. Curr. Opin. Chem. Biol. 18, 8–15 (2014).
Furst, A. L., Smith, M. J. & Francis, M. B. New techniques for the generation and analysis of tailored microbial systems on surfaces. Biochemistry 57, 3017–3026 (2018).
Vinkenborg, J. L., Mayer, G. & Famulok, M. Aptamer-based affinity labeling of proteins. Angew. Chem. Int. Ed. 51, 9176–9180 (2012).
Cui, C. et al. Recognition-then-reaction enables site-selective bioconjugation to proteins on live-cell surfaces. Angew. Chem. Int. Ed. 56, 11954–11957 (2017).
Li, L. et al. Aptamer displacement reaction from live-cell surfaces and its applications. J. Am. Chem. Soc. 141, 17174–17179 (2019).
Wang, R. et al. Using modified aptamers for site specific protein–aptamer conjugations. Chem. Sci. 7, 2157–2161 (2016).
Robinson, P. V., de Almeida-Escobedo, G., de Groot, A. E., McKechnie, J. L. & Bertozzi, C. R. Live-cell labeling of specific protein glycoforms by proximity-enhanced bioorthogonal ligation. J. Am. Chem. Soc. 137, 10452–10455 (2015).
Skovsgaard, M. B., Mortensen, M. R., Palmfeldt, J. & Gothelf, K. V. Aptamer-directed conjugation of DNA to therapeutic antibodies. Bioconjugate Chem. 30, 2127–2135 (2019).
Tamura, T. & Hamachi, I. Chemistry for covalent modification of endogenous/native proteins: from test tubes to complex biological systems. J. Am. Chem. Soc. 141, 2782–2799 (2019).
Li, G., Liu, Y., Chen, L., Wu, S. & Li, X. Photoaffinity labeling of small-molecule-binding proteins by DNA-templated chemistry. Angew. Chem. Int. Ed. 52, 9544–9549 (2013).
Wang, D. Y. et al. Target identification of kinase inhibitor alisertib (MLN8237) by using DNA-programmed affinity labeling. Chem. Eur. J. 23, 10906–10914 (2017).
Liu, Y. et al. Photoaffinity labeling of transcription factors by DNA-templated crosslinking. Chem. Sci. 6, 745–751 (2015).
Bai, X. et al. Development of a DNA-templated peptide probe for photoaffinity labeling and enrichment of the histone modification reader proteins. Angew. Chem. Int. Ed. 55, 7993–7997 (2016).
Bai, X. et al. An integrated approach based on a DNA self-assembly technique for characterization of crosstalk among combinatorial histone modifications. Anal. Chem. 90, 3692–3696 (2018).
Rosen, C. B. et al. Template-directed covalent conjugation of DNA to native antibodies, transferrin and other metal-binding proteins. Nat. Chem. 6, 804–809 (2014).
Kodal, A. L., Rosen, C. B., Mortensen, M. R., Torring, T. & Gothelf, K. V. DNA-templated introduction of an aldehyde handle in proteins. ChemBioChem 17, 1338–1342 (2016).
Kane, M. A. et al. Influence on immunoreactive folate-binding proteins of extracellular folate concentration in cultured human cells. J. Clin. Invest. 81, 1398–1406 (1988).
Furst, A. L., Klass, S. H. & Francis, M. B. DNA hybridization to control cellular interactions. Trends Biochem. Sci 44, 342–350 (2019).
Kim, C., Ye, F. & Ginsberg, M. H. Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 27, 321–345 (2011).
Zhang, D. Y. & Winfree, E. Control of DNA strand displacement kinetics using toehold exchange. J. Am. Chem. Soc. 131, 17303–17314 (2009).
Li, G. et al. Design, preparation, and selection of DNA-encoded dynamic libraries. Chem. Sci. 6, 7097–7104 (2015).
Miki, T. et al. LDAI-based chemical labeling of intact membrane proteins and its pulse-chase analysis under live cell conditions. Chem. Biol. 21, 1013–1022 (2014).
Weber, G. Optimization method for obtaining nearest-neighbour DNA entropies and enthalpies directly from melting temperatures. Bioinformatics 31, 871–877 (2015).
Fujishima, S. H., Yasui, R., Miki, T., Ojida, A. & Hamachi, I. Ligand-directed acyl imidazole chemistry for labeling of membrane-bound proteins on live cells. J. Am. Chem. Soc. 134, 3961–3964 (2012).
Mizusawa, K., Takaoka, Y. & Hamachi, I. Specific cell surface protein imaging by extended self-assembling fluorescent turn-on nanoprobes. J. Am. Chem. Soc. 134, 13386–13395 (2012).
Coyle, M. P., Xu, Q., Chiang, S., Francis, M. B. & Groves, J. T. DNA-mediated assembly of protein heterodimers on membrane surfaces. J. Am. Chem. Soc. 135, 5012–5016 (2013).
Wrenn, S. J., Weisinger, R. M., Halpin, D. R. & Harbury, P. B. Synthetic ligands discovered by in vitro selection. J. Am. Chem. Soc. 129, 13137–13143 (2007).
Hansen, M. H. et al. A yoctoliter-scale DNA reactor for small-molecule evolution. J. Am. Chem. Soc. 131, 1322–1327 (2009).
Milo, R., Jorgensen, P., Moran, U., Weber, G. & Springer, M. BioNumbers–the database of key numbers in molecular and cell biology. Nucleic Acids Res. 38, D750–D753 (2010).
Rijnboutt, S. et al. Endocytosis of GPI-linked membrane folate receptor-ɑ. J. Cell Biol. 132, 35–47 (1996).
Wong, P. T. & Choi, S. K. Mechanisms and implications of dual-acting methotrexate in folate-targeted nanotherapeutic delivery. Int. J. Mol. Sci. 16, 1772–1790 (2015).
Vauquelin, G. & Charlton, S. J. Exploring avidity: understanding the potential gains in functional affinity and target residence time of bivalent and heterobivalent ligands. Br. J. Pharmacol. 168, 1771–1785 (2013).
Brown, D. G. et al. Agonists and antagonists of protease-activated receptor 2 discovered within a DNA-encoded chemical library using mutational stabilization of the target. SLAS Discov. 23, 429–436 (2018).
Kibbe, W. A. OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res. 35, W43–W46 (2007).
Clark, M. A. et al. Design, synthesis and selection of DNA-encoded small-molecule libraries. Nat. Chem. Biol. 5, 647–654 (2009).
Kleiner, R. E., Dumelin, C. E., Tiu, G. C., Sakurai, K. & Liu, D. R. In vitro selection of a DNA-templated small-molecule library reveals a class of macrocyclic kinase inhibitors. J. Am. Chem. Soc. 132, 11779–11791 (2010).
Mannocci, L. et al. High-throughput sequencing allows the identification of binding molecules isolated from DNA-encoded chemical libraries. Proc. Natl Acad. Sci. USA 105, 17670–17675 (2008).
Jiang, R. D., Shen, H. & Piao, Y. J. The morphometrical analysis on the ultrastructure of A549 cells. Rom. J. Morphol. Embryol. 51, 663–667 (2010).
Zhang, F. et al. Quantification of epidermal growth factor receptor expression level and binding kinetics on cell surfaces by surface plasmon resonance imaging. Anal. Chem. 87, 9960–9965 (2015).
Li, Y., Zhao, P., Zhang, M., Zhao, X. & Li, X. Multistep DNA-templated synthesis using a universal template. J. Am. Chem. Soc. 135, 17727–17730 (2013).
Gartner, Z. J. et al. DNA-templated organic synthesis and selection of a library of macrocycles. Science 305, 1601–1605 (2004).
Usanov, D. L., Chan, A. I., Maianti, J. P. & Liu, D. R. Second-generation DNA-templated macrocycle libraries for the discovery of bioactive small molecules. Nat. Chem. 10, 704–714 (2018).
Acknowledgements
This work was supported by grants from the Research Grants Council of the Hong Kong Special Administrative Region, China (AoE/P-705/16, 17321916, 17302817, 17301118, 17111319 and 17303220), Laboratory for Synthetic Chemistry and Chemical Biology of Health@InnoHK of ITC, HKSAR, National Natural Science Foundation of China (21572014, 21877093, 81603067, 21907011 and 91953119), the Fundamental Research Funds for the Central Universities (project numbers 2019CDQYYX018 and 2020CQJQY-Z002), Chongqing Research and Frontier Technology (cstc2020jcyj-jqX0009) and Venture & Innovation Support Program for Chongqing Overseas Returnees (cx2019084) for Y.L. We thank the Centre for PanorOmic Sciences (CPOS) Genomics Core at HKU for NGS support and the CQU-Agilent Joint Lab on DNA-encoded Library for MS support.
Author information
Authors and Affiliations
Contributions
Y.H., Y.L. and Xiaoyu Li conceived and designed the experiments. Y.H., L.M., Q.N., Y.Z., L.C., S.Y., Xiaomeng Li and C.H. carried out the experiments and analysed the data. Y.M.E.F. carried out the MS experiments and analysis. Y.C. designed and carried out the SPR experiments and analysis. Y.H., L.M., Q.N., Y.Z., L.C., S.Y., Y.M.E.F., Xiaomeng Li, C.H., Y.C., Y.L. and Xiaoyu Li co-wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Labelling of CA-12 on live cells using CBS-guided BP/CP probes.
a) Structures of CBS-BP, NC-BP (a negative binding probe), and CP-1. A549 cells were labelled with CBS-BP/CP-1 and NC-BP (no CBS)/CP-1, respectively, targeting CA-12 on the cell surface. Experimental conditions are the same as in Fig. 2 of the main text. b)-c) Flow cytometry analysis of the labelled A549 cells. d) Column graph summarizing the flow cytometry results. e) CBS-BP and NC-BP were paired with biotin-CP-1, respectively, and used to label CA-12 on A549 and MCF-7 cells. After labelling, the cells were lysed and the biotinylated proteins were analysed with western blotting. M: marker; lanes 1 and 3: with CBS-BP; lanes 2 and 4: with NC-BP. *: endogenous biotinylated proteins. Loading control: internal endogenous biotinylated proteins, marked with *; a portion of each sample was separately blotted for CA-12 and actin as additional input/sample processing controls. In d), n = 3 biologically independent samples were measured; data are presented as mean values ± SD (standard deviation).
Extended Data Fig. 2 Additional data on toehold displacement of BP after the labelling of FR on HeLa cells.
a) Structures of BP-2 and CP-2 and the labelling scheme. Fluorescent imaging and flow cytometry were used to monitor a series of control experiments. b) Toehold displacement with a mismatched DP after labelling. Unlike the complementary DP shown in Fig. 3, the mismatched DP did not reduce cell fluorescence. c)-e) After toehold displacement with a complementary DP to remove the original BP, a series of control experiments were performed. c): with no FA on BP-3; d): with a mismatched BP-3; e): with free FA competitor (50-fold). Experimental conditions were the same as in Fig. 2 of the main text. f) The same labelling and toehold displacement experiments were performed with the CBS/CA-12 system. Flow cytometry histograms after labelling and before/after DP displacement are shown. Strong fluorescence reduction was observed.
Extended Data Fig. 3 Preparation of antibody–DNA conjugates.
a) The conjugation reaction scheme. b) Native PAGE analysis of the reaction. Lane 1, an anti-EGFR antibody standard; lane 2: the reaction mixture. Marker is based on unmodified antibody. c) The bands a–c in b) were purified and analysed with native PAGE (polyacrylamide gel electrophoresis). d) The unlabelled antibody and purified bands a–c were characterized with ESI-MS; the results confirmed that conjugate b was the mono-DNA–antibody conjugate.
Extended Data Fig. 4 DEL selection against HeLa cells with different FR expression levels.
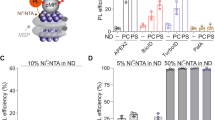
a) Besides FA, another FR ligand, methotrexate (MTX) was conjugated with a DNA strand and added to the 4,800-member library as shown in Fig. 5. This 4,802-member DEL was selected against the DNA-tagged FR on HeLa cells with different FR levels; a tag with 7-nt complementarity was used in the selections. b) FP analysis to measure the binding affinity of the MTX–DNA conjugate to the target protein FR, and a Kd of ~26.6 µM was obtained. n = 3 biologically independent FP samples were measured. Data are presented as mean values ± SD (standard deviation) based on biologically independent replicates. c) HeLa cells were cultured in FA-deficient medium and harvested after different passages. Six batches of the cells with different FR expression levels were fluorescently labelled with BP-1/CP-1 and analysed with flow cytometry for each batch. The average number of DNA molecules on each cell was measured; based on a size of 3,000 cubic µm for HeLa cells, the FR concentration for each cell batch was calculated (200 µL selection volume; see Section 9 for calculation method). The 4,802-member DEL was subjected to the same selection procedure as described in Fig. 5,6 against these cell batches, respectively; the selection results were processed also in the same way and summarized in the table. EF: enrichment factor. For each selection, a control without FR tagging was also conducted. d) Column graph summarizing the selection results shown in c).
Extended Data Fig. 5 DEL selection against HeLa cells labelled with the tags with different lengths of complementary bases.
a) The 4,802-member DEL was selected against the DNA-tagged FR on HeLa cells with different lengths of complementary bases in the tag. Two cell batches (P1 and P4) were used in the selections. The selection procedure and data processing method were the same as in Figs. 5–6. b) The effects of different DNA tag lengths were calculated and summarized in the table; key parameters include: ΔH, ΔS, ΔG, fold of affinity increase, and Kd of the DNA tag/library DNA duplex. c)–d) Column graph and the table summarizing the selection results; EF: enrichment factor. The tag lengths from 6 to 10 bases corresponded to a free energy gain from 5.65 to 9.70 kcal/mol and an affinity increase of ~11,000 to 9-million folds. At 6- and 7-nt, the tag hybridized with library DNA at µM affinity; at 9- and 10-nt, the tag and the library DNA formed stable duplexes, which would increase the affinity of all library compounds to nM binders (Kd: 285 nM and 103 nM, respectively). The results also showed that the enrichment fold of FA and MTX dropped with the 10-nt tag, but many other library compounds were enriched. We reasoned this might be because the 10-nt tag formed stable DNA duplex with all library compounds and resulted in the enrichment of many low-affinity binders. DNA tag shorter than 6-base was not tested because it would have hybridization specificity issue at such a short length; the tag may hybridize with the other regions of the library DNA, instead of the primer-binding site.
Extended Data Fig. 6 DEL selection against the DNA-tagged CA-12 on live cells.
a) Structures of GLCBS–DNA and CBS–DNA, which were two positive controls added to the 4,800-member DEL for selection against CA-12 on A549 cells. b) Scatter plots of the selection results of the tagged A549 cells (top) and the untagged cells (bottom). The selection experiment condition and data processing protocol are the same as in Fig. 7. x-axis: post-sequencing counts; y-axis: enrichment fold = (post-selection %)/(pre-selection %) of each compound. The positive controls (GLCBS and CBS) are highlighted. c) Calculation of the DNA-tagged CA-12 concentration on A549 cells. The average number of DNA on each cell was determined with flow cytometry.
Supplementary information
Supplementary Information
Supplementary Figs. 1–33, Tables 1–3, discussion, detailed experimental procedures, additional discussion and other additional information.
Supplementary Table 1
Source data for Supplementary Figures.
Supplementary Table 2
Source data for mass spectrometry analysis.
Supplementary Software 1
Custom code for data analysis.
Source data
Source Data Fig. 2
Full-length, unprocessed western blots and gels.
Source Data Fig. 2
Statistical Source Data.
Source Data Fig. 4
Full-length, unprocessed western blots and gels.
Source Data Fig. 4
Statistical Source Data.
Source Data Fig. 5
Statistical Source Data.
Source Data Fig. 6
Statistical Source Data.
Source Data Fig. 7
Statistical Source Data.
Source Data Extended Data Fig. 1
Full-length, unprocessed western blots and gels.
Source Data Extended Data Fig. 1
Statistical Source Data.
Source Data Extended Data Fig. 3
Full-length, unprocessed western blots and gels.
Source Data Extended Data Fig. 4
Statistical Source Data.
Source Data Extended Data Fig. 5
Statistical Source Data.
Source Data Extended Data Fig. 6
Statistical Source Data.
Rights and permissions
About this article
Cite this article
Huang, Y., Meng, L., Nie, Q. et al. Selection of DNA-encoded chemical libraries against endogenous membrane proteins on live cells. Nat. Chem. 13, 77–88 (2021). https://doi.org/10.1038/s41557-020-00605-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00605-x