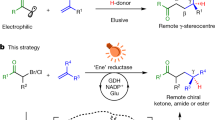
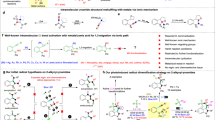
Abstract
Oxidative cyclizations create many unique chemical structures that are characteristic of biologically active natural products. Many of these reactions are catalysed by ‘non-canonical’ or ‘thwarted’ iron oxygenases and appear to involve long-lived radicals. Mimicking these biosynthetic transformations with chemical equivalents has been a long-standing goal of synthetic chemists but the fleeting nature of radicals, particularly under oxidizing conditions, makes this challenging. Here we use redox-neutral photocatalysis to generate radicals that are likely to be involved in the biosynthesis of lignan natural products. We present the total syntheses of highly oxidized dibenzocyclooctadienes, which feature densely fused, polycyclic frameworks that originate from a common radical progenitor. We show that multiple factors control the fate of the proposed biosynthetic radicals, as they select between 5- or 11-membered ring cyclizations and a number of different terminating events. Our syntheses create new opportunities to explore the medicinal properties of these natural products, while shedding light on their biosynthetic origin.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study, including characterization data for all compounds produced in this work, are included in this published article and its Supplementary Information files. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 1916278 (22), 1916279 (S17), 1916280 (8) and 2026509 (46). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Xu, L. J., Liu, H. T., Peng, Y. & Xiao, P.-G. A preliminary pharmacophylogenetic investigation in Schisandraceae. J. Syst. Evol. 46, 692–723 (2008).
Panossian, A. & Wikman, G. Pharmacology of Schisandra chinensis Bail.: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 118, 183–212 (2008).
Liu, J. et al. Genus Kadsura, a good source with considerable characteristic chemical constituents and potential bioactivities. Phytomedicine 21, 1092–1097 (2014).
Chang, J., Reiner, J. & Xie, J. Progress on the chemistry of dibenzocyclooctadiene lignans. Chem. Rev. 105, 4581–4609 (2005).
Han, Y.-S. et al. Identification of a dibenzocyclooctadiene lignan as a HIV-1 non-nucleoside reverse transcriptase inhibitor. Antivir. Chem. Chemother. 24, 28–38 (2015).
Zhu, P., Li, J., Fu, X. & Yu, Z. Schisandra fruits for the management of drug-induced liver injury in China: A review. Phytomedicine 59, 152760 (2019).
Lau, W. & Sattely, E. S. Six enzymes from mayapple that complete the biosynthetic pathway to the etoposide aglycone. Science 349, 1224–1228 (2015).
Davin, L. B. et al. Dissection of lignin macromolecular configuration and assembly: Comparison to related biochemical processes in allyl/propenyl phenol and lignan biosynthesis. Nat. Prod. Rep. 25, 1015–1090 (2008).
Ikeya, Y., Taguchi, H. & Yosioka, I. The constituents of Schizandra chinensis BAILL. The structures of two new lignans, pre-gomisin and gomisin. J. Chem. Pharm. Bull. 26, 682–684 (1978).
Lin, Y.-C. et al. New lignans from the leaves and stems of Kadsura philippinensis. Molecules 18, 6573–6583 (2013).
Liu, J. & Huang, M. Kadsulignans E-G from Kadsura longipedunculata. Phytochemistry 31, 957–960 (1992).
Xu, L.-J., Peng, Y., Chen, S.-B., Chen, S.-L. & Xiao, P.-G. Four new lignans from Kadsura heteroclita. Heterocycles 71, 941–947 (2007).
Shen, Y.-C., Lin, Y.-C., Cheng, Y.-B., Kuo, Y.-H. & Liaw, C.-C. Taiwankadsurins A, B, and C, three new C19 homolignans from Kadsura philippinensis. Org. Lett. 7, 5297–5300 (2005).
Tang, M.-C., Zou, Y., Watanabe, K., Walsh, C. T. & Tang, Y. Oxidative cyclization in natural product biosynthesis. Chem. Rev. 117, 5226–5333 (2017).
Walsh, C. T. & Moore, B. S. Enzymatic cascade reactions in biosynthesis. Angew. Chem. Int. Ed. 58, 6846–6879 (2019).
Huang, X. & Groves, J. T. Oxygen activation and radical transformations in heme proteins and metalloporphyrins. Chem. Rev. 118, 2491–2553 (2018).
Okada, K., Okamoto, K. & Oda, M. A new and practical method of decarboxylation: photosensitized decarboxylation of N-acyloxyphthalimides via electron-transfer mechanism. J. Am. Chem. Soc. 110, 8736–8738 (1988).
Okada, K., Okamoto, K., Morita, N., Okubo, K. & Oda, M. Photosensitized decarboxylative Michael addition through N-(acyloxy)phthalimides via an electron-transfer mechanism. J. Am. Chem. Soc. 113, 9401–9402 (1991).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Shaw, M. H., Twilton, J. & MacMillan, D. W. C. Photoredox catalysis in organic chemistry. J. Org. Chem. 81, 6898–6926 (2016).
Gong, W. & RajanBabu, T. V. Conformation and reactivity in dibenzocyclooctadienes (DBCOD). A general approach to the total synthesis of fully substituted DBCOD lignans via borostannylative cyclization of α,ω-diynes. Chem. Sci. 4, 3979–3985 (2013).
Bringmann, G., Gulder, T., Gulder, T. A. M. & Breuning, M. Atroposelective total synthesis of axially chiral biaryl natural products. Chem. Rev. 111, 563–639 (2011).
DeMartino, M. P. The Design and Development of Oxidative Enolate Heterocoupling and Application Toward the Total Synthesis of the Taiwankadsurins. PhD thesis, The Scripps Research Institute (2008).
Tomioka, K., Ishiguro, T., Iitaka, Y. & Koga, K. Asymmetric total synthesis of natural (–)- and unnatural (+)-steganacin: Determination of the absolute configuration of natural antitumor steganacin. Tetrahedron 40, 1303–1312 (1984).
Coleman, R. S., Gurrala, S. R., Mitra, S. & Raao, A. Asymmetric total synthesis of dibenzocyclooctadiene lignan natural products. J. Org. Chem. 70, 8932–8941 (2005).
Belokon, Y. N. et al. Mechanism-guided development of VO(salen)X complexes as catalysts for the asymmetric synthesis of cyanohydrin trimethylsilyl ethers. Chem. Eur. J. 15, 2148–2165 (2009).
Van Draanen, N. A., Arseniyadis, S., Crimmins, M. T. & Heathcock, C. H. Protocols for the preparation of each of the four possible stereoisomeric α-alkyl-β-hydroxy carboxylic acids from a single chiral aldol reagent. J. Org. Chem. 56, 2499–2506 (1991).
Galobardes, M. et al. Enolization of chiral α-silyloxy ketones with dicyclohexylchloroborane. Application to stereoselective aldol reactions. Org. Lett. 2, 2599–2602 (2000).
Aullón, G., Romea, P. & Urpí, F. Substrate-controlled aldol reactions from chiral α-hydroxy ketones. Synthesis 49, 484–503 (2017).
Bartoli, G., Bosco, M., Di Martino, E., Marcantoni, E. & Sambri, L. Highly stereoselective and efficient addition of organocerium reagents to syn-β-alkyl-β-hydroxy-α-methyl ketones by way of their titanium alkoxides − synthesis of complex 1,3-diol units with three stereodefined centres. Eur. J. Org. Chem. 2001, 2901–2909 (2001).
Alam, A., Takaguchi, Y. & Tsuboi, S. 1,3-Dibromo-5,5-dimethylhydantoin, a useful reagent for ortho-monobromination of phenols and polyphenols. J. Fac. Environ. Sci. Technol., Okayama Univ. 10, 105–109 (2005).
Huang, Z. & Lumb, J.-P. Phenol-directed C–H functionalization. ACS Catal. 9, 521–555 (2019).
Kinzel, T., Zhang, Y. & Buchwald, S. L. A new palladium precatalyst allows for the fast Suzuki−Miyaura coupling reactions of unstable polyfluorophenyl and 2-heteroaryl boronic acids. J. Am. Chem. Soc. 132, 14073–14075 (2010).
Patel, N. D. et al. Computationally assisted mechanistic investigation and development of Pd-catalyzed asymmetric Suzuki–Miyaura and Negishi cross-coupling reactions for tetra-ortho-substituted biaryl synthesis. ACS Catal. 8, 10190–10209 (2018).
Thomas, A. A. & Denmark, S. E. Pre-transmetalation intermediates in the Suzuki-Miyaura reaction revealed: The missing link. Science 352, 329–332 (2016).
Carrow, B. P. & Hartwig, J. F. Distinguishing between pathways for transmetalation in Suzuki−Miyaura reactions. J. Am. Chem. Soc. 133, 2116–2119 (2011).
Lawlor, D. A. et al. Hyperaromatic stabilization of arenium ions: cyclohexa- and cycloheptadienyl cations—experimental and calculated stabilities and ring currents. J. Am. Chem. Soc. 133, 19729–19742 (2011).
Tlahuext-Aca, A., Garza-Sanchez, R. A. & Glorius, F. Multicomponent oxyalkylation of styrenes enabled by hydrogen-bond-assisted photoinduced electron transfer. Angew. Chem. Int. Ed. 56, 3708–3711 (2017).
Pratsch, G., Lackner, G. L. & Overman, L. E. Constructing quaternary carbons from N-(acyloxy)phthalimide precursors of tertiary radicals using visible-light photocatalysis. J. Org. Chem. 80, 6025–6036 (2015).
Pham, P. V., Nagib, D. A. & MacMillan, D. W. C. Photoredox catalysis: A mild, operationally simple approach to the synthesis of α-trifluoromethyl carbonyl compounds. Angew. Chem. Int. Ed. 50, 6119–6122 (2011).
Firn, R. D. & Jones, C. G. Natural products – a simple model to explain chemical diversity. Nat. Prod. Rep. 20, 382–391 (2003).
Yet, L. in Privileged Structures in Drug Discovery (ed. Yet, L.) Ch. 4, 83–154 (Wiley, 2018).
Genovino, J., Lütz, S., Sames, D. & Touré, B. B. Complementation of biotransformations with chemical C–H oxidation: copper-catalyzed oxidation of tertiary amines in complex pharmaceuticals. J. Am. Chem. Soc. 135, 12346–12352 (2013).
Green, S. P. & Whiting, D. A. New carbon radical chemistry as a model for the biogenesis of the interiorin/kadsulignan type of dibenzocyclooctadiene lignan. J. Chem. Soc., Perkin Trans. 1, 193–202 (1998).
Coleman, R. S., Guernon, J. M. & Roland, J. T. Synthesis of the spirocyclic cyclohexadienone ring system of the schiarisanrins. Org. Lett. 2, 277–280 (2000).
Ikeya, Y., Taguchi, H. & Iitaka, Y. The constituents of Schizandra chinensis Baill. The structure of a new lignan, gomisin D. Tetrahedron Lett. 17, 1359–1362 (1976).
Yang, G.-Y. et al. Bioactive lignans from the leaves and stems of Schisandra wilsoniana. Nat. Prod. Commun. 8, 467–470 (2013).
Liu, J., Huang, M. & Zhou, H. Kadsulignan C and D, two novel lignans from Kadsura longipedunculata. Can. J. Chem. 69, 1403–1407 (1991).
Wang, G.-Z., Shang, R. & Fu, Y. Irradiation-induced palladium-catalyzed decarboxylative heck reaction of aliphatic N-(acyloxy)phthalimides at room temperature. Org. Lett. 20, 888–891 (2018).
Crossley, S. W. M., Obradors, C., Martinez, R. M. & Shenvi, R. A. Mn-, Fe-, and Co-catalyzed radical hydrofunctionalizations of olefins. Chem. Rev. 116, 8912–9000 (2016).
Satoshi, I., Koji, K., Shigeru, I. & Teruaki, M. A new and facile method for the direct preparation of α-hydroxycarboxylic acid esters from α,β-unsaturated carboxylic acid esters with molecular oxygen and phenylsilane catalyzed by bis(dipivaloylmethanato)manganese(ii) complex. Chem. Lett. 19, 1869–1872 (1990).
Newcomb, M. Competition methods and scales for alkyl radical reaction kinetics. Tetrahedron 49, 1151–1176 (1993).
Shen, Y.-C. et al. Kadsuphilols A-H, oxygenated lignans from Kadsura philippinensis. J. Nat. Prod. 70, 1139–1145 (2007).
Wertjes, W. C., Southgate, E. H. & Sarlah, D. Recent advances in chemical dearomatization of nonactivated arenes. Chem. Soc. Rev. 47, 7996–8017 (2018).
Flynn, A. R., McDaniel, K. A., Hughes, M. E., Vogt, D. B. & Jui, N. T. Hydroarylation of arenes via reductive radical-polar crossover. J. Am. Chem. Soc. 142, 9163–9168 (2020).
Smith, J. M., Harwood, S. J. & Baran, P. S. Radical retrosynthesis. Acc. Chem. Res. 51, 1807–1817 (2018).
Acknowledgements
We thank T. Maris from the University of Montreal for help with X-ray crystallography and N. Moitessier and J. Plescia from McGill University for help with ozonolysis. Financial support was provided by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant to J.-P.L.), the Fonds de Recherche du Québec Nature et Technologies (FRQNT) (Team Grant to J.-P.L.) and the FRQNT Center for Green Chemistry and Catalysis.
Author information
Authors and Affiliations
Contributions
Z.H. and J.-P.L. conceived and designed the experiments. Z.H. performed the experiments. Z.H. and J.-P.L. analysed the data. Z.H. and J.-P.L. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
The Supplementary Information contains Supplementary Figs. 1–14, Discussion and Tables 1–12 associated with the manuscript, as well as procedural details for and characterization data of all newly prepared compounds, including their NMR spectra and, where appropriate, their X-ray data
Supplementary Data 1
Crystallographic data for compound 8. CCDC reference 1916280
Supplementary Data 2
Crystallographic data for compound 22. CCDC reference 1916278
Supplementary Data 3
Crystallographic data for compound 46. CCDC reference 2026509
Supplementary Data 4
Crystallographic data for compound S17. CCDC reference 1916279
Rights and permissions
About this article
Cite this article
Huang, Z., Lumb, JP. Mimicking oxidative radical cyclizations of lignan biosynthesis using redox-neutral photocatalysis. Nat. Chem. 13, 24–32 (2021). https://doi.org/10.1038/s41557-020-00603-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00603-z
This article is cited by
-
Making light work of lignan synthesis
Nature Chemistry (2021)