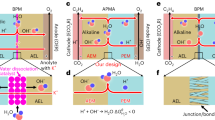
Abstract
The efficient conversion of electricity to chemicals is needed to mitigate the intermittency of renewable energy sources. Driving these electrochemical conversions at useful rates requires not only fast electrode kinetics, but also rapid mass and ion transport. However, little is known about the effect of local environments on ionic flows in solid polymer electrolytes. Here, we show that it is possible to measure and manipulate the local pH in membrane electrolysers with a resolution of tens of nanometres. In bipolar-membrane-based gas-fed CO2 electrolysers, the acidic environment of the cation exchange layer results in low CO2 reduction efficiency. By using ratiometric indicators and layer-by-layer polyelectrolyte assembly, the local pH was measured and controlled within an ~50-nm-thick weak-acid layer. The weak-acid layer suppressed the competing hydrogen evolution reaction without affecting CO2 reduction. This method of probing and controlling the local membrane environment may be useful in devices such as electrolysers, fuel cells and flow batteries, as well as in operando studies of ion distributions within polymer electrolytes.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are included in this paper and its Supplementary Information and Source Data files. Source data are provided with this paper.
References
Seh, Z. W. et al. Combining theory and experiment in electrocatalysis: insights into materials design. Science 355, eaad4998 (2017).
Lewis, N. S. & Nocera, D. G. Powering the planet: chemical challenges in solar energy utilization. Proc. Natl Acad. Sci. USA 103, 15729–15735 (2006).
Yan, Z., Hitt, J. L., Turner, J. A. & Mallouk, T. E. Renewable electricity storage using electrolysis. Proc. Natl Acad. Sci. USA 117, 12558–12563 (2020).
Mogensen, M. B. et al. Reversible solid-oxide cells for clean and sustainable energy. Clean Energy 3, 175–201 (2019).
Hori, Y. in Modern Aspects of Electrochemistry (eds Vayenas, C. G. et al.) 89–189 (Springer, 2008).
Weng, L. C., Bell, A. T. & Weber, A. Z. Modeling gas-diffusion electrodes for CO2 reduction. Phys. Chem. Chem. Phys. 20, 16973–16984 (2018).
Delacourt, C., Ridgway, P. L., Kerr, J. B. & Newman, J. Design of an electrochemical cell making syngas (CO + H2) from CO2 and H2O reduction at room temperature. J. Electrochem. Soc. 155, B42–B49 (2008).
Vennekoetter, J. B., Sengpiel, R. & Wessling, M. Beyond the catalyst: how electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem. Eng. J. 364, 89–101 (2019).
Ma, S. et al. One-step electrosynthesis of ethylene and ethanol from CO2 in an alkaline electrolyzer. J. Power Sources 301, 219–228 (2016).
Dinh, C. T. et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface. Science 360, 783–787 (2018).
Li, Y. C. et al. Bipolar membranes inhibit product crossover in CO2 electrolysis cells. Adv. Sustain. Syst. 2, 1700187 (2018).
Ünlü, M., Zhou, J. & Kohl, P. A. Hybrid anion and proton exchange membrane fuel cells. J. Phys. Chem. C 113, 11416–11423 (2009).
Vargas‐Barbosa, N. M., Geise, G. M., Hickner, M. A. & Mallouk, T. E. Assessing the utility of bipolar membranes for use in photoelectrochemical water-splitting cells. ChemSusChem 7, 3017–3020 (2014).
Luo, J. et al. Bipolar membrane-assisted solar water splitting in optimal pH. Adv. Energy Mater. 6, 1600100 (2016).
Sun, K. et al. A stabilized, intrinsically safe, 10% efficient, solar-driven water-splitting cell incorporating earth-abundant electrocatalysts with steady-state pH gradients and product separation enabled by a bipolar membrane. Adv. Energy Mater. 6, 1600379 (2016).
McDonald, M. B., Ardo, S., Lewis, N. S. & Freund, M. S. Use of bipolar membranes for maintaining steady-state pH gradients in membrane-supported, solar-driven water splitting. ChemSusChem 7, 3021–3027 (2014).
McDonald, M. B., Freund, M. S. & Hammond, P. T. Catalytic, conductive bipolar membrane interfaces through layer-by-layer deposition for the design of membrane-integrated artificial photosynthesis systems. ChemSusChem 10, 4599–4609 (2017).
Li, Y. C. et al. Electrolysis of CO2 to syngas in bipolar membrane-based electrochemical cells. ACS Energy Lett. 1, 1149–1153 (2016).
Salvatore, D. A. et al. Electrolysis of gaseous CO2 to CO in a flow cell with a bipolar membrane. ACS Energy Lett. 3, 149–154 (2018).
Yang, H., Kaczur, J. J., Sajjad, S. D. & Masel, R. I. Electrochemical conversion of CO2 to formic acid utilizing SustainionTM membranes. J. CO2 Util. 20, 208–217 (2017).
Xia, C. et al. Continuous production of pure liquid fuel solutions via electrocatalytic CO2 reduction using solid-electrolyte devices. Nat. Energy 4, 776–785 (2019).
Peterson, A. A., Abild-Pedersen, F., Studt, F., Rossmeisl, J. & Nørskov, J. K. How copper catalyzes the electroreduction of carbon dioxide into hydrocarbon fuels. Energy Environ. Sci. 3, 1311–1315 (2010).
Kortlever, R., Shen, J., Schouten, K. J. P., Calle-Vallejo, F. & Koper, M. T. M. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide. J. Phys. Chem. Lett. 6, 4073–4082 (2015).
Hansen, H. A., Varley, J. B., Peterson, A. A. & Nørskov, J. K. Understanding trends in the electrocatalytic activity of metals and enzymes for CO2 reduction to CO. J. Phys. Chem. Lett. 4, 388–392 (2013).
Yang, K., Kas, R. & Smith, W. A. In-situ infrared spectroscopy reveals persistent alkalinity near electrode surfaces during CO2 electroreduction. J. Am. Chem. Soc. 141, 15891–15900 (2019).
Dunwell, M. et al. Examination of near-electrode concentration gradients and kinetic impacts on the electrochemical reduction of CO2 using surface-enhanced infrared spectroscopy. ACS Catal. 8, 3999–4008 (2018).
Ryu, J. & Surendranath, Y. Tracking electrical fields at the Pt/H2O interface during hydrogen catalysis. J. Am. Chem. Soc. 141, 15524–15531 (2019).
Ryu, J., Wuttig, A. & Surendranath, Y. Quantification of interfacial pH variation at molecular length scales using a concurrent non-Faradaic reaction. Angew. Chem. Int. Ed. 57, 9300–9304 (2018).
Han, J. & Burgess, K. Fluorescent indicators for intracellular pH. Chem. Rev. 110, 2709–2728 (2010).
Kang, Z. et al. Investigation of thin/well-tunable liquid/gas diffusion layers exhibiting superior multifunctional performance in low-temperature electrolytic water splitting. Energy Environ. Sci. 10, 166–175 (2017).
Yan, Z. et al. The balance of electric field and interfacial catalysis in promoting water dissociation in bipolar membranes. Energy Environ. Sci. 11, 2235–2245 (2018).
Li, Y. C. et al. CO2 electroreduction from carbonate electrolyte. ACS Energy Lett. 4, 1427–1431 (2019).
Helfferich, F. G. Ion Exchange (Courier Corporation, 1995).
Decher, G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 277, 1232–1237 (1997).
Abdu, S., Martí-Calatayud, M. C., Wong, J. E., García-Gabaldón, M. & Wessling, M. Layer-by-layer modification of cation exchange membranes controls ion selectivity and water splitting. ACS Appl. Mater. Interfaces 6, 1843–1854 (2014).
White, N., Misovich, M., Yaroshchuk, A. & Bruening, M. L. Coating of Nafion membranes with polyelectrolyte multilayers to achieve high monovalent/divalent cation electrodialysis selectivities. ACS Appl. Mater. Interfaces 7, 6620–6628 (2015).
Abdu, S. et al. Catalytic polyelectrolyte multilayers at the bipolar membrane interface. ACS Appl. Mater. Interfaces 5, 10445–10455 (2013).
Lin, H.-J., Szmacinski, H. & Lakowicz, J. R. Lifetime-based pH sensors: indicators for acidic environments. Anal. Biochem. 269, 162–167 (1999).
Johnson, I. & Spence, M. (eds) Molecular Probes Handbook: A Guide to Fluorescent Probes and Labelling Technologies 11th edn (Life Technologies, 2010).
Kaschak, D. M. et al. Photoinduced energy and electron transfer reactions in lamellar polyanion/polycation thin films: toward an inorganic ‘leaf’. J. Am. Chem. Soc. 121, 3435–3445 (1999).
Nikonenko, V. V. & Kozmai, A. E. Electrical equivalent circuit of an ion-exchange membrane system. Electrochim. Acta 56, 1262–1269 (2011).
Zamel, N. The catalyst layer and its dimensionality—a look into its ingredients and how to characterize their effects. J. Power Sources 309, 141–159 (2016).
Huang, J., Li, Z. & Zhang, J. Review of characterization and modeling of polymer electrolyte fuel cell catalyst layer: the blessing and curse of ionomer. Front. Energy 11, 334–364 (2017).
Wuttig, A., Yaguchi, M., Motobayashi, K., Osawa, M. & Surendranath, Y. Inhibited proton transfer enhances Au-catalyzed CO2-to-fuels selectivity. Proc. Natl Acad. Sci. USA 113, E4585–E4593 (2016).
Acknowledgements
Z.Y. thanks L. Ren for assisting with sample preparation. This work was supported by the Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences and Energy Bioscience, Department of Energy (contract no. SC-0019781) and by the Canadian Institute for Advanced Research. J.L.H. acknowledges support as a graduate fellow of the Vagelos Institute for Energy Science and Technology at the University of Pennsylvania. Anion exchange membrane synthesis was supported by the DOE-NETL University Coalition for Fossil Energy Research (UCFER) (grant no. DE-FE0026825, subaward 0001-PSU-DOE-6825).
Author information
Authors and Affiliations
Contributions
Z.Y., M.A.H. and T.E.M. designed the study. Z.Y. carried out the experimental work with assistance from J.L.H. and Z.Z. Z.Y. and T.E.M. drafted the manuscript, which was reviewed and edited by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–14 and Discussion.
Source data
Source Data Fig. 1
Statistical source data for Fig. 1b.
Source Data Fig. 2
Statistical source data for Fig. 2b.
Source Data Fig. 4
Statistical source data for Fig. 4.
Source Data Fig. 5
Statistical source data for Fig. 5.
Rights and permissions
About this article
Cite this article
Yan, Z., Hitt, J.L., Zeng, Z. et al. Improving the efficiency of CO2 electrolysis by using a bipolar membrane with a weak-acid cation exchange layer. Nat. Chem. 13, 33–40 (2021). https://doi.org/10.1038/s41557-020-00602-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00602-0
This article is cited by
-
Multi-scale physics of bipolar membranes in electrochemical processes
Nature Chemical Engineering (2024)
-
Durable CO2 conversion in the proton-exchange membrane system
Nature (2024)
-
Continuous ammonia electrosynthesis using physically interlocked bipolar membrane at 1000 mA cm−2
Nature Communications (2023)
-
Coverage-driven selectivity switch from ethylene to acetate in high-rate CO2/CO electrolysis
Nature Nanotechnology (2023)
-
Conversion of CO2 to multicarbon products in strong acid by controlling the catalyst microenvironment
Nature Synthesis (2023)