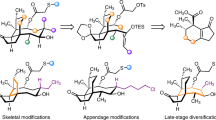
Abstract
Polyether ionophores are complex natural products capable of transporting cations across biological membranes. Many polyether ionophores possess potent antimicrobial activity and a few selected compounds have the ability to target aggressive cancer cells. Nevertheless, ionophore function is believed to be associated with idiosyncratic cellular toxicity and, consequently, human clinical development has not been pursued. Here, we demonstrate that structurally novel polyether ionophores can be efficiently constructed by recycling components of highly abundant polyethers to afford analogues with enhanced antibacterial selectivity compared to a panel of natural polyether ionophores. We used classic degradation reactions of the natural polyethers lasalocid and monensin and combined the resulting fragments with building blocks provided by total synthesis, including halogen-functionalized tetronic acids as cation-binding groups. Our results suggest that structural optimization of polyether ionophores is possible and that this area represents a potential opportunity for future methodological innovation.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
MIC and IC50 values for cell viability for each biological replicate are available in Supplementary Table 1. The morphological profiling data (aggregated profiles and correlation matrix), included in Supplementary Figs. 3–9, have been deposited at Mendeley Data (https://doi.org/10.17632/jv3sjk8wy4.2). The images from morphological profiling are too large to be deposited at Mendeley Data (which has a limit of 10 GB per data set), but can be obtained upon request. The X-ray crystallography data have been deposited in the Cambridge Crystallographic Data Centre (CCDC) using the following identifiers (www.ccdc.cam.ac.uk/structures/): 1920656 (6-Na), 1920657 (14), 1920658 (24), 1920659 (29-Na) and 1920660 (31-Na).
Code availability
Code/scripts for analysis of morphological profiling data and Cell Profiler pipelines have been deposited at Mendeley Data (https://doi.org/10.17632/jv3sjk8wy4.2).
References
Huffman, B. J. & Shenvi, R. A. Natural products in the ‘marketplace’: interfacing synthesis and biology. J. Am. Chem. Soc. 141, 3332–3346 (2019).
Schreiber, S. L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 287, 1964–1969 (2000).
Wetzel, S., Bon, R. S., Kumar, K. & Waldmann, H. Biology-oriented synthesis. Angew. Chem. Int. Ed. 50, 10800–10826 (2011).
Könst, Z. A. et al. Synthesis facilitates an understanding of the structural basis for translation inhibition by the lissoclimides. Nat. Chem. 9, 1140–1149 (2017).
Wilson, R. M. & Danishefsky, S. J. Small molecule natural products in the discovery of therapeutic agents: the synthesis connection. J. Org. Chem. 71, 8329–8351 (2006).
Huigens, R. W. et al. A ring-distortion strategy to construct stereochemically complex and structurally diverse compounds from natural products. Nat. Chem. 5, 195–202 (2013).
Abbasov, M. E. et al. Simplified immunosuppressive and neuroprotective agents based on gracilin A. Nat. Chem. 11, 342–350 (2019).
Karageorgis, G., Foley, D. J., Laraia, L. & Waldmann, H. Principle and design of pseudo-natural products. Nat. Chem. 12, 227–235 (2020).
Seiple, I. B. et al. A platform for the discovery of new macrolide antibiotics. Nature 533, 338–345 (2016).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Westley, J. (ed.) Polyether Antibiotics—Naturally Occurring Acid Ionophores 1st edn (Marcel Dekker, 1982).
Dutton, C. J., Banks, B. J. & Cooper, C. B. Polyether ionophores. Nat. Prod. Rep. 12, 165–181 (1995).
Nakata, T. et al. A total synthesis of lasalocid A. J. Am. Chem. Soc. 100, 2933–2935 (1978).
Fukuyama, T. et al. Total synthesis of monensin. 3. Stereocontrolled total synthesis of monensin. J. Am. Chem. Soc. 101, 262–263 (1979).
Faul, M. M. & Huff, B. E. Strategy and methodology development for the total synthesis of polyether ionophore antibiotics. Chem. Rev. 100, 2407–2473 (2000).
Song, Z., Lohse, A. G. & Hsung, R. P. Challenges in the synthesis of a unique mono-carboxylic acid antibiotic, (+)-zincophorin. Nat. Prod. Rep. 26, 560–571 (2009).
Kasun, Z. A., Gao, X., Lipinski, R. M. & Krische, M. J. Direct generation of triketide stereopolyads via merged redox-construction events: total synthesis of (+)-zincophorin methyl ester. J. Am. Chem. Soc. 137, 8900–8903 (2015).
Wang, G. & Krische, M. J. Total synthesis of (+)-SCH 351448: efficiency via chemoselectivity and redox-economy powered by metal catalysis. J. Am. Chem. Soc. 138, 8088–8091 (2016).
Chen, L.-A., Ashley, M. A. & Leighton, J. L. Evolution of an efficient and scalable nine-step (longest linear sequence) synthesis of zincophorin methyl ester. J. Am. Chem. Soc. 139, 4568–4573 (2017).
Liu, H., Lin, S., Jacobsen, K. M. & Poulsen, T. B. Chemical syntheses and chemical biology of carboxyl polyether ionophores: recent highlights. Angew. Chem. Int. Ed. 58, 13630–13642 (2019).
Kevin, D. A. II, Meujo, D. A. & Hamann, M. T. Polyether ionophores: broad-spectrum and promising biologically active molecules for the control of drug-resistant bacteria and parasites. Expert Opin. Drug Discov. 4, 109–146 (2009).
Chapman, H. D., Jeffers, T. K. & Williams, R. B. Forty years of monensin for the control of coccidiosis in poultry. Poultry Sci. 89, 1788–1801 (2010).
Goodrich, R. D. et al. Influence of monensin on the performance of cattle. J. Anim. Sci. 58, 1484–1498 (1984).
Antoszczak, M. et al. Biological activity of doubly modified salinomycin analogs—evaluation in vitro and ex vivo. Eur. J. Med. Chem. 156, 510–523 (2018).
Igarashi, Y. et al. Nonthmicin, a polyether polyketide bearing a halogen-modified tetronate with neuroprotective and antiinvasive activity from Actinomadura sp. Org. Lett. 19, 1406–1409 (2017).
Wyche, T. P. et al. Chemical genomics, structure elucidation, and in vivo studies of the marine-derived anticlostridial ecteinamycin. ACS Chem. Biol. 12, 2287–2295 (2017).
Westley, J. W., Evans, R. H., Williams, T. & Stempel, A. Structure of antibiotic X-537A. Chem. Commun. 71–72 (1970).
Westley, J. W., Evans, R. H., Williams, T. & Stempel, A. Pyrolytic cleavage of antibiotic x-537A and related reactions. J. Org. Chem. 38, 3431–3433 (1973).
Gruenfeld, N. et al. Angiotensin converting enzyme inhibitors: 1-glutarylindoline-2-carboxylic acids derivatives. J. Med. Chem. 26, 1277–1282 (1983).
Hiyama, T., Kimura, K. & Nozaki, H. Chromium(ii) mediated threo selective synthesis of homoallyl alcohols. Tetrahedron Lett. 22, 1037–1040 (1981).
Hansen, T. M. et al. Highly chemoselective oxidation of 1,5-diols to δ-lactones with TEMPO/BAIB. Tetrahedron Lett. 44, 57–59 (2003).
Brazeau, J.-F. et al. Stereocentrolled synthesis of C1–C17 fragment of narasin via a free radical-based approach. Org. Lett. 12, 36–39 (2010).
Zografos, A. L. & Georgiadis, D. Synthetic strategies towards naturally occurring tetronic acids. Synthesis 3157–3188 (2006).
Markó, I. E., Richardson, P. R., Bailey, M., Maguire, A. R. & Coughlan, N. Selective manganese-mediated transformations using the combination: KMnO4/Me3SiCl. Tetrahedron Lett. 38, 2339–2342 (1997).
Sabbah, M., Bernollin, M., Doutheau, A., Soulère, L. & Queneau, Y. A new route towards fimbrolide analogues: importance of the exomethylene motif in LuxR dependent quorum sensing inhibition. Med. Chem. Commun. 4, 363–366 (2013).
Roush, W. R. Concerning the diastereofacial selectivity of the aldol reactions of α-methyl chiral aldehydes and lithium and boron propionate enolates. J. Org. Chem. 56, 4151–4157 (1991).
Masamune, S., Choy, W., Petersen, J. S. & Sita, L. R. Double asymmetric synthesis and a new strategy for stereochemical control in organic synthesis. Angew. Chem. Int. Ed. 24, 1–30 (1985).
Evans, D. A., Yang, M. G., Dart, M. J., Duffy, J. L. & Kim, A. S. Double stereodifferentiating lewis acid-promoted (Mukaiyama) aldol bond constructions. J. Am. Chem. Soc. 117, 9598–9599 (1995).
Nicolaou, K. C., Estrada, A. A., Zak, M., Lee, S. H. & Safina, B. S. A mild and selective method for the hydrolysis of esters with trimethyltin hydroxide. Angew. Chem. Int. Ed. 44, 1378–1382 (2005).
Vögtle, F. & Weber, E. Multidentate acyclic neutral ligands and their complexation. Angew. Chem. Int. Ed. 18, 753–776 (1979).
Evans, D., Chapman, K. & Carreira, E. M. Directed reduction of β-hydroxy ketones employing tetramethylammonium triacetoxylborohydride. J. Am. Chem. Soc. 110, 3560–3578 (1988).
Cai, D. & Still, W. C. Synthesis of monensin. Reconstruction from degradation products. J. Org. Chem. 53, 4641–4643 (1988).
Baskaran, S., Islam, I., Raghaven, M. & Chandrasekaran, S. Pyridinium chlorochromate in organic synthesis. A facile and selective oxidative cleavage of enol ethers. Chem. Lett. 16, 1175–1178 (1987).
Bray, M.-A. et al. Cell painting, a high-content image-based assay for morphological profiling using multiplexed fluorescent dyes. Nat. Protoc. 11, 1757–1774 (2016).
Svenningsen, E. B. & Poulsen, T. B. Establishing cell painting in a smaller chemical biology lab—a report from the frontier. Bioorg. Med. Chem. 27, 2609–2615 (2019).
Hutz, J. E. et al. The multidimensional perturbation value: a single metric to measure similarity and activity of treatments in high-throughput multidimensional screens. J. Biomol. Screen. 18, 367–377 (2013).
Golbek, T. W., Schmüsen, L., Rasmussen, M. H., Poulsen, T. B. & Weidner, T. Lasalocid acid antibiotic at a membrane surface probed by sum frequency generation spectroscopy. Langmuir 36, 3184–3192 (2020).
Versini, A. et al. Chemical biology of salinomycin. Tetrahedron 74, 5585–5614 (2018).
Mai, T. T. et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 9, 1025–1033 (2017).
Wang, F. et al. Nucleolin is a functional binding protein for salinomycin in neuroblastoma stem cells. J. Am. Chem. Soc. 141, 3613–3622 (2019).
Heathcock, C. H. & Flippin, L. A. Acyclic stereoselection. 16. High diastereofacial selectivity in Lewis acid mediated additions of enol silanes to chiral aldehydes. J. Am. Chem. Soc. 105, 1667–1668 (1983).
Lamprecht, M. R., Sabatini, D. M. & Carpenter, A. E. CellProfilerTM: free, versatile software for automated biological image analysis. Biotechniques 42, 71–75 (2007).
Becker, T., Goodman, A., McQuin, C., Rohban, M. & Singh, S. cytominer: methods for image-based cell profiling. R package v.0.1.0 (2017); https://cran.r-project.org/package=cytominer
R Core Team. R: a language and environment for statistical computing (R Foundation for Statistical Computing, 2017); https://www.R-project.org/
Warnes, G. R. et al. gplots: various R programming tools for plotting data. R package v.3.0.3 (2020); https://cran.r-project.org/package=gplots
Wei, T. & Simko, V. R package ‘corrplot’: visualization of a correlation matrix. R package v.0.84 (2017); https://github.com/taiyun/corrplot
Acknowledgements
This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 865738). Financial support from the Carlsberg Foundation (grant CF17-0800), Novo Nordisk Foundation (grant NNF19OC0054782) and Independent Research Fund Denmark (grants 9040-00117B and 6110-00600B) is acknowledged. We thank E. Jung and A. Johnbeck for technical assistance with the organic syntheses, A. Bodholt Nielsen for technical assistance with NMR spectroscopy, and I.C. Stensgaard Jensen for technical assistance with microbiology.
Author information
Authors and Affiliations
Contributions
T.B.P. conceived and supervised the study. T.B.P., S.L., H.L. and E.B.S. designed the experiments. S.L., H.L., J.M.V. and C.N.P. performed the organic syntheses. E.B.S. and K.M.J. conducted the cell biology experiments and analysed data. M.W. and F.D.A. conducted the microbiology experiments. T.T. supervised the microbiology experiments and analysed data. P.N. carried out the X-ray crystallographic analyses. T.B.P., S.L., H.L. and E.B.S. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Strategies for the synthesis of protected 5-(halo)methylidene tetronic acid building blocks and subsequent coupling.
a, Chemical structure of the targeted 3-acyltetronic acid-derivatives found in nonthmicin/ecteinamycin and 6. No previous syntheses of the halogenated variants have been reported. b, Examples of known methods used to prepare non-halogenated variants. c, Failed attempts and model studies toward 5-chloromethylidene tetronate. I) Unsuccessful synthesis of 5-tributylstannylmethylidene tetronate. II) Unsuccessful chlorination of 5-dimethylaminomethylidene tetronate. III) Direct and indirect chlorination of 5-methylidene tetronate methyl ether. In practice, the removal of the methyl group from the chlorinated tetronate product using LiCl/DMSO or BBr3 was difficult. Thus, we moved to synthesize the tetronate derivatives bearing a TMSE group, which can be removed under much milder condition (TBAF/DMF). DMSO = dimethylsulfoxide DCC = N,N’-dicyclohexylcarbodiimide, DMAP = 4-dimethylaminopyridine, Ms = methanesulfonyl, MOM = methoxymethyl, DCE = 1,2-dichloroethane, DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, TMS = trimethylsilyl, NCS = N-chlorosuccinimide, AIBN = azobisisobutyronitrile.
Extended Data Fig. 2 Synthesis of hybrid polyether HL324 (32).
Preparation of analogue HL324 (32) was performed through anti-selective (Evans-Saksena) reduction of the ketone-functionality of 26 followed by DCC-mediated coupling with 5-chloromethylidene tetronate. rt = room temperature, TBAF = tetrabutylammonium fluoride, DMF = N,N-dimethylformamide, DCC = N,N’-dicyclohexylcarbodiimide, DMAP = 4-dimethylaminopyridine, HPLC = high pressure liquid chromatography.
Extended Data Fig. 3 Droplet screen for antibacterial activity in B. cereus.
a, Representative images of inhibition zones of all compounds from B. cereus. b, Heatmap representing no (red), slight (yellow) or large (green) inhibition zones. All compounds were tested at 10 mM in DMSO except monensin (1 mM) and HL160 (7 mM). c) Chemical structures of acid-containing synthetic fragments. SL382 = 27; SL415 = 26 – see Fig. 3.
Extended Data Fig. 4 Use of morphological profiling via cell painting to determine bioactivity threshold in U-2OS cells.
a, Plots of cell count (grey) and Mahalanobis distance (‘Bioactivity’; red and green) against concentration of compound. Green points indicate significant activity (mp-value < 0.01) while red points indicate no significant activity (mp-value > 0.01). Many ionophores induce an active profile without loss of cell viability while bioactivity of the hybrid ionophores is correlated with a loss of cells, indicating a profile representing toxicity. The bioactivity threshold is determined as the first concentration to reach significance (mp-value < 0.01). b, Dose-dependent morphological profiles from which the bioactivity threshold is determined. Note that some ionophores change profile at higher concentrations, for example calcimycin (Cal) and nanchangmycin (Nan), typically correlated with a loss of cells. See Supplementary Fig. 3–9 for representative images, all dose-response plots, profiles and correlation matrices in both U-2OS and Vero cells.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, X-ray crystallography methods and comments, morphological profiling imaging settings, synthesis protocols and characterization data, references and NMR spectra.
Supplementary Table 1
Complete data from MIC determinations and cell viability experiments.
Supplementary Data 1
Cif file for compound 6-Na.
Supplementary Data 2
Structure factors file for compound 6-Na.
Supplementary Data 3
Cif file for compound 14.
Supplementary Data 4
Structure factors file for compound 14.
Supplementary Data 5
Cif file for compound 24.
Supplementary Data 6
Structure factors file for compound 24.
Supplementary Data 7
Cif file for compound 29-Na.
Supplementary Data 8
Structure factors file for compound 29-Na.
Supplementary Data 9
Cif file for compound 31-Na.
Supplementary Data 10
Structure factors file for compound 31-Na.
Rights and permissions
About this article
Cite this article
Lin, S., Liu, H., Svenningsen, E.B. et al. Expanding the antibacterial selectivity of polyether ionophore antibiotics through diversity-focused semisynthesis. Nat. Chem. 13, 47–55 (2021). https://doi.org/10.1038/s41557-020-00601-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00601-1