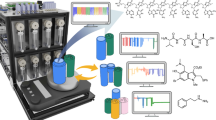
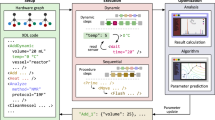
Abstract
Although the automatic synthesis of molecules has been established, each reaction class uses bespoke hardware. This means that the connection of multi-step syntheses in a single machine to run many different protocols and reactions is not possible, as manual intervention is required. Here we show how the Chemputer synthesis robot can be programmed to perform many different reactions, including solid-phase peptide synthesis, iterative cross-coupling and accessing reactive, unstable diazirines in a single, unified system with high yields and purity. Developing universal and modular hardware that can be automated using one software system makes a wide variety of batch chemistry accessible. This is shown by our system, which performed around 8,500 operations while reusing only 22 distinct steps in 10 unique modules, with the code able to access 17 different reactions. We also demonstrate a complex convergent robotic synthesis of a peptide reacted with a diazirine—a process requiring 12 synthetic steps.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data are available in the supplementary volume. This includes full experimental details to build the Chemputer as well as compound characterization.
Code availability
The code to run the hardware for the automated platforms and the scripts to run the reactions are available in the supplementary volume and in the open-source repository (https://github.com/croningp/ChemputerConvergence).
Change history
24 January 2024
In the version of the article initially published, the Supplementary Software file was corrupted. This has now been replaced.
References
Peplow, M. Organic synthesis: the robo-chemist. Nature 512, 20–22 (2014).
Davies, I. W. The digitization of organic synthesis. Nature 570, 175–181 (2019).
Merrifield, R. B. Automated synthesis of peptides. Science 150, 178–185 (1965).
Alvarado-Urbina, G. et al. Automated synthesis of gene fragments. Science 214, 270–274 (1981).
Plante, O. J., Palmacci, E. R. & Seeberger, P. H. Automated solid-phase synthesis of oligosaccharides. Science 291, 1523–1527 (2001).
Li, J. et al. Synthesis of many different types of organic small molecules using one automated process. Science 347, 1221–1226 (2015).
Coley, C. W. et al. A robotic platform for flow synthesis of organic compounds informed by AI planning. Science 365, eaax1566 (2019).
Adamo, A. et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 352, 61–67 (2016).
Chatterjee, S., Guidi, M., Seeberger, P. H. & Gilmore, K. Automated radial synthesis of organic molecules. Nature 579, 379–384 (2020).
Steiner, S. et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363, eaav2211 (2019).
Merrifield, R. B., Stewart, J. M. & Jernberg, N. Instrument for automated synthesis of peptides. Anal. Chem. 38, 1905–1914 (1966).
Kates, S. A., Daniels, S. B. & Albericio, F. Automated allyl cleavage for continuous-flow synthesis of cyclic and branched peptides. Anal. Biochem. 212, 303–310 (1993).
Jensen, K. J., Shelton, P. T. & Pedersen, S. L. Peptide Synthesis and Applications (Humana Press, 2013).
Zheng, H. et al. An automated Teflon microfluidic peptide synthesizer. Lab Chip 13, 3347 (2013).
Iyer, L. K., Moorthy, B. S. & Topp, E. M. Photolytic cross-linking to probe protein–protein and protein–matrix interactions in lyophilized powders. Mol. Pharm. 12, 3237–3249 (2015).
Cheng, X. et al. Light-triggered assembly of gold nanoparticles for photothermal therapy and photoacoustic imaging of tumors in vivo. Adv. Mater. 29, 1604894 (2017).
Matsuguma, H. J. & Audrieth, L. F. The stability of aqueous solutions of hydroxylamine-O-sulphonic acid. J. Inorg. Nucl. Chem. 12, 186–192 (1959).
Mehr, S. H. M., Craven, M., Leonov, A. I., Keenan, G. & Cronin, L. A universal system for digitization and automatic execution of the chemical synthesis literature. Science 370, 101–108 (2020).
Acknowledgements
We thank BUCHI for supplying us with an R-300 rotary evaporator and an API to interface it with the Chemputer software package, D. Castro for help with the NHS-diazirine peptide synthesis and purity assessment, H. Mehr for Python advice on the conductivity sensor development and A. Jones for suggesting the diazirine synthesis challenge. We also thank M. Symes, P. Kitson and N. Bell for comments on the manuscript as well as N. A. B. Johnson for her artistic depiction of the Chemputer platform in the TOC graphic. We thank the following funders: EPSRC (Grant Nos. EP/H024107/1, EP/J00135X/1, EP/J015156/1, EP/K021966/1, EP/K023004/1, EP/L023652/1), ERC (project 670467 SMART-POM). This research was developed with funding from the Defense Advanced Research Projects Agency (DARPA). The views, opinions and/or findings expressed are those of the authors and should not be interpreted as representing the official views or policies of the Department of Defense or the US Government.
Author information
Authors and Affiliations
Contributions
L.C. conceived the concept, the selection of the chemistry, the architecture, outline module design, and the programming approach. D.A., A.J.S.H., S.R., S.K., J.M.G. and J.W. helped configure the robots, run the synthetic protocols and characterize the products. The new modules were designed and built by D.A., A.J.S.H., S.R. and S.Z., and the construction manuals were compiled by G.C. L.C. wrote the paper together with D.A., A.J.S.H. and S.R. with help from all the authors.
Corresponding author
Ethics declarations
Competing interests
L.C. is the founder of DeepMatter Group PLC and Chemify Ltd., which aims to commercialize various aspects of the digitization of chemistry, including discovery and synthesis using universal robotic platforms.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Angelone, D., Hammer, A.J.S., Rohrbach, S. et al. Convergence of multiple synthetic paradigms in a universally programmable chemical synthesis machine. Nat. Chem. 13, 63–69 (2021). https://doi.org/10.1038/s41557-020-00596-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00596-9
This article is cited by
-
Universal chemical programming language for robotic synthesis repeatability
Nature Synthesis (2024)
-
Autonomous execution of highly reactive chemical transformations in the Schlenkputer
Nature Chemical Engineering (2024)
-
An integrated self-optimizing programmable chemical synthesis and reaction engine
Nature Communications (2024)
-
A robotic platform for the synthesis of colloidal nanocrystals
Nature Synthesis (2023)
-
Using the pandemic as a driver for innovation in research
Nature Reviews Methods Primers (2022)