Abstract
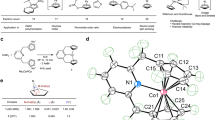
The discovery of ferrocene nearly 70 years ago marked the genesis of metallocene chemistry. Although the ferrocenium cation was discovered soon afterwards, a derivatized ferrocenium dication was only isolated in 2016 and the monoanion of ferrocene has only been observed in low-temperature electrochemical studies. Here we report the isolation of a derivatized ferrocene anion in the solid state as part of an isostructural family of 3d metallocenates, which consist of anionic complexes of a metal centre (manganese, iron or cobalt) sandwiched between two bulky Cpttt ligands (where Cpttt is {1,2,4-C5H2 tBu3}). These thermally and air-sensitive complexes decompose rapidly above −30 °C; however, we were able to characterize all metallocenates by a wide range of physical techniques and ab initio calculations. These data have allowed us to map the electronic structures of this metallocenate family, including an unexpected high-spin S = 3/2 ground state for the 19e− derivatized ferrocene anion.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition nos. CCDC 1951767 (1), 1951768 (2), 1951769 (3), 1951770 (4), 1951771 (5), 1951772 (6), 1951773 (7) and 1951774 (8). Copies of the data can be obtained free of charge from the CCDC via www.ccdc.cam.ac.uk/structures. Raw research data files supporting this publication are available from Mendeley Data at https://doi.org/10.17632/rzzpcwgkx5.1. Apart from the datasets mentioned, all other data supporting the findings of this study are available within the Article and Supplementary Information.
References
Kealy, T. J. & Pauson, P. L. A new type of organo-iron compound. Nature 168, 1039–1040 (1951).
Miller, S. A., Tebboth, J. A. & Tremaine, J. F. 114. Dicyclopentadienyliron. J. Chem. Soc. 1952, 632–635 (1952).
Wilkinson, G., Rosenblum, M., Whiting, M. C. & Woodward, R. B. The structure of iron bis-cyclopentadienyl. J. Am. Chem. Soc. 74, 2125–2126 (1952).
Long, N. J. Metallocenes—An Introduction to Sandwich Complexes (Blackwell Science, 1998).
Togni, A. & Halterman, R. L. Metallocenes: Synthesis Reactivity Applications (Wiley, 1998).
Fukino, T. et al. Manipulation of discrete nanostructures by selective modulation of noncovalent forces. Science 344, 499–540 (2014).
Astruc, D. Why is ferrocene so exceptional?. Eur. J. Inorg. Chem. 2017, 6–29 (2017).
Jumde, R. P., Lanza, F., Veenstra, M. J. & Harutyunyan, S. R. Catalytic asymmetric addition of Grignard reagents to alkenyl-substituted aromatic N-heterocycles. Science 352, 433–437 (2016).
Connelly, N. G. & Geiger, W. E. Jr Chemical redox agents for organometallic chemistry. Chem. Rev. 96, 877–910 (1996).
Malischewski, M., Adelhardt, M., Sutter, J., Meyer, K. & Seppelt, K. Isolation and structural and electronic characterization of salts of the decamethylferrocene dication. Science 353, 678–682 (2016).
Holloway, J. D. L., Bowden, W. L. & Geiger, W. E. Jr Unusual electron-transfer processes involving electron-rich and electron-deficient metallocenes. J. Am. Chem. Soc. 99, 7089–7090 (1997).
Mugnier, Y., Moise, C., Tirouflet, J. & Laviron, E. Reduction electrochimique du ferrocene. J. Organomet. Chem. 186, C49–C52 (1980).
Ito, N., Saji, T. & Aoyagui, S. Electrochemical formation of stable ferrocene anion and the formal rate constant of the ferrocene0/− electrode. J. Organomet. Chem. 247, 301–305 (1983).
Geiger, W. E. Jr Electroreduction of cobaltocene. Evidence for a metallocene anion. J. Am. Chem. Soc. 96, 2632–2634 (1974).
Bard, A. J., Garcia, E., Kukharenko, S. & Strelets, V. V. Electrochemistry of metallocenes at very negative and very positive potentials. Electrogeneration of 17-electron Cp2Co2+, 21-electron Cp2Co2−, and 22-electron Cp2Ni2− species. Inorg. Chem. 32, 3528–3531 (1993).
Smart, J. C. & Robbins, J. L. A low spin manganocene and its novel anionic derivative. Synthesis and characterization of decamethylmanganocene complexes. J. Am. Chem. Soc. 100, 3936–3937 (1978).
Robbins, J. L., Edelstein, N. M., Cooper, S. R. & Smart, J. C. Syntheses and electronic structures of decamethylmanganocenes. J. Am. Chem. Soc. 101, 3853–3857 (1979).
Baudry, D. & Ephritikhine, M. Reactions de (η5-C5H5)2reh et de ses derives. Preparation de nouveaux complexes biscyclopentadienyles du rhenium. J. Organomet. Chem. 195, 213–222 (1980).
Gardner, B. M., McMaster, J., Lewis, W. & Liddle, S. T. Synthesis and structure of [{N(CH2CH2NSiMe3)3}URe(η5-C5H5)2]: a heterobimetallic complex with an unsupported uranium-rhenium bond. Chem. Commun. 2009, 2851–2853 (2009).
Hung-Low, F. & Bradley, C. A. Synthesis of a bis(indenyl) Co(i) anion: a reactive source of a 14 electron indenyl Co(i) equivalent. Inorg. Chem. 52, 2446–2457 (2013).
Malischewski, M. & Seppelt, K. Structural characterization of potassium salts of the decamethylmanganocene anion Cp*2Mn−. Dalton Trans. 48, 17078–17082 (2019).
Astruc, D., Román, E., Hamon, E., Batail, J. R. & Novel, P. Reactions of dioxygen in organometallic chemistry. Hydrogen atom abstraction vs. dimerization of the 19-electron complexes η5-cyclopentadienyliron(i) η6-arene. J. Am. Chem. Soc. 101, 2240–2242 (1979).
Hamon, J. R., Astruc, D. & Michaud, P. Syntheses, characterizations, and stereoelectronic stabilization of organometallic electron reservoirs: the 19-electron d7 redox catalysts η5-C5R5Fe-η6-C6R′6. J. Am. Chem. Soc. 103, 758–766 (1981).
Saito, M. et al. Anionic stannaferrocene and its unique electronic state. Chem. Lett. 48, 163–165 (2019).
Sitzmann, H., Schär, M., Dormann, E. & Kelemen, M. High spin-manganocenes with bulky, alkylated cyclopentadienyl ligands. Z. Anorg. Allg. Chem. 623, 1609–1613 (1997).
Walter, M. D., Sofield, C. D., Booth, C. H. & Andersen, R. A. Spin equilibria in monomeric manganocenes: solid-state magnetic and EXAFS studies. Organometallics 28, 2005–2019 (2009).
Walter, M. D. & White, P. S. [Cp′FeI]2 as convenient entry into iron-modified pincer complexes: bimetallic η6,κ1-POCOP-pincer iron iridium compounds. New J. Chem. 35, 1842–1854 (2011).
Schneider, J. J. et al. Synthesis, structure and spectroelectrochemistry of bis(η6-1,4-tri-tert-butyl-benzene)chromium(0) and bis(η5-1,2,4-tri-tert-butyl-cyclopentadienyl)cobalt(ii). Dia- and paramagnetic sandwich complexes derived from sterically highly demanding π-ligands. J. Organomet. Chem. 590, 7–14 (1999).
Peters, M. et al. Pogo-stick iron and cobalt complexes: synthesis, structures, and magnetic properties. Inorg. Chem. 58, 16475–16486 (2019).
Jaroschik, F., Nief, F., Le Goff, X.-F. & Ricard, L. Synthesis and reactivity of organometallic complexes of divalent thulium with cyclopentadienyl and phospholyl ligands. Organometallics 26, 3552–3558 (2007).
Woen, D. H. & Evans, W. J. in Handbook on the Physics and Chemistry of Rare Earths Vol. 50 (eds Bünzli, J.-C. G. & Pecharsky, V. K.) Ch. 293, 337–394 (Elsevier, 2016).
Fischer, E. O. & Leipfinger, H. Weitere magnetische Untersuchungen zur Struktur der cyclopentadien- und inden-Verbindungen der Übergangsmetalle. Z. Naturforsch. 10b, 353–355 (1955).
Wilkinson, G., Cotton, F. A. & Birmingham, J. M. On manganese cyclopentadienide and some chemical reactions of neutral bis-cyclopentadienyl metal compounds. J. Inorg. Nucl. Chem. 2, 95–113 (1956).
Walter, M. D., Sofield, C. D. & Andersen, R. A. Spin equilibria and thermodynamic constants for (C5H4R)2Mn, R = H or Me, in solid solutions of diamagnetic diluents. J. Organomet. Chem. 776, 17–22 (2015).
Switzer, M. E., Wang, R., Rettig, M. F. & Maki, A. H. Electronic ground states of manganocene and 1,1′-dimethylmanganocene. J. Am. Chem. Soc. 96, 7669–7674 (1974).
Fdez. Galván, I. et al. OpenMolcas: from source code to insight. J. Chem. Theory Comput. 15, 5925–5964 (2019).
Wickman, H. H., Klein, M. P. & Shirley, D. A. Paramagnetic hyperfine structure and relaxation effects in Mössbauer spectra: Fe57 in ferrichrome A. Phys. Rev. 152, 345 (1966).
Stoian, S. A. et al. Mössbauer, electron paramagnetic resonance, and crystallographic characterization of a high-spin Fe(i) diketiminate complex with orbital degeneracy. Inorg. Chem. 44, 4915–4922 (2005).
Wertheim, G. K. & Herber, R. H. Fe57 Mössbauer effect in ferrocene derivatives. J. Chem. Phys. 38, 2106–2111 (1963).
Stukan, R. A., Gubin, S. P., Nesmeyanov, A. N., Gol’danskii, V. I. & Makarov, E. F. A Mössbauer study of some ferrocene derivatives. Theor. Exper. Chem. 2, 581–584 (1966).
Reiners, M. et al. Study on spin–lattice relaxation processes in tert-butyl substituted ferrocenium derivatives. Eur. J. Inorg. Chem. 388–400 (2017).
Mariot, J. P. et al. Mössbauer study and molecular orbital calculations on the organo-iron(i, ii) electron reservoir sandwiches CpFen+ (η6-C6(CH3)6) (n = 0,1) and related CpFe (cyclohexadienyl) complexes. J. Physique 44, 1377–1385 (1983).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Chilton, N. F., Anderson, R. P., Turner, L. D., Soncini, A. & Murray, K. S. PHI: a powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 34, 1164–1175 (2013).
Rajasekharan, M. V. et al. EPR studies of the electronic structure and dynamic Jahn–Teller effect in iron(i) sandwich compounds. J. Am. Chem. Soc. 104, 2400–2407 (1982).
Chang, H.-C. et al. Electron paramagnetic resonance signature of tetragonal low spin iron(v)–nitrido and –oxo complexes derived from the electronic structure analysis of heme and non-heme archetypes. J. Am. Chem. Soc. 141, 2421–2434 (2019).
Gomez-Coca, S., Cremades, E., Aliaga-Alcalde, N. & Ruiz, E. Mononuclear single-molecule magnets: tailoring the magnetic anisotropy of first-row transition–metal complexes. J. Am. Chem. Soc. 135, 7010–7018 (2013).
Molloy, K. C. (ed.) in Group Theory for Chemists 2nd edn, Ch. 10, 109–118 (Woodhead Publishing, 2013); https://doi.org/10.1533/9780857092410.3.109
Cirera, J. & Ruiz, E. Electronic and steric control of the spin-crossover behavior in [(CpR)2Mn] manganocenes. Inorg. Chem. 57, 702–709 (2018).
Ishimura, K., Hada, M. & Nakatsuji, H. Ionized and excited states of ferrocene: symmetry adapted cluster–configuration–interaction study. J. Chem. Phys. 117, 6533–6537 (2002).
Watt, G. W. & Baye, L. J. Reactions of metallocenes with potassium and potassium amide in liquid ammonia. J. Inorg. Nucl. Chem. 26, 2099–2102 (1964).
Bergbreiter, D. E. & Killough, J. M. Reactions of potassium-graphite. J. Am. Chem. Soc. 100, 2126–2134 (1978).
Roos, B. O., Lindh, R., Malmqvist, P.-Å., Veryazov, V. & Widmark, P.-O. Main group atoms and dimers studied with a new relativistic ANO basis set. J. Phys. Chem. A 108, 2851–2858 (2004).
Roos, B. O., Lindh, R., Malmqvist, P.-Å., Veryazov, V. & Widmark, P.-O. New relativistic ANO basis sets for transition metal atoms. J. Phys. Chem. A 109, 6575–6579 (2005).
Veryazov, V., Malmqvist, P. Å. & Roos, B. O. How to select active space for multiconfigurational quantum chemistry? Int. J. Quant. Chem. 111, 3329–3338 (2011).
Acknowledgements
We acknowledge funding from the Engineering and Physical Sciences Research Council (Doctoral Prize Fellowship to C.A.P.G., EP/N007034/1 for M.V., EP/R002605X/1 for P.E., studentship for H.M.N. and EP/K039547/1 for a single-crystal X-ray diffractometer), the Royal Society (University Research Fellowship to N.F.C.), European Research Council CoG-816268 (D.P.M. and M.J.G.) and StG-851504 (N.F.C.) and the University of Manchester (Presidential Doctoral Prize to M.J.G.). C.A.P.G. and S.M.G. thank the Laboratory Directed Research and Development (LDRD) programme at Los Alamos National Laboratory (an affirmative action/equal opportunity employer, managed by Triad National Security, LLC, for the NNSA of the US Department of Energy) (contract no. 89233218CNA000001) for a distinguished J. Robert Oppenheimer Postdoctoral Fellowship and Directors Fellowship, respectively. S.H. acknowledges support from the National Science Foundation (DMR-1610226). We thank the EPSRC UK National Electron Paramagnetic Resonance Service for access to the EPR Facility, and the University of Manchester for access to the Computational Shared Facility. A portion of this work was performed at the National High Magnetic Field Laboratory, which is supported by the National Science Foundation Cooperative agreement no. DMR-1644779 and the State of Florida. We also thank F. Ortu for assistance with the collection of Raman spectra. This project has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreements nos. 816268 and 851504).
Author information
Authors and Affiliations
Contributions
C.A.P.G. and D.P.M. provided the original concept. C.A.P.G. synthesized and characterized the compounds. H.M.N. and P.E. carried out supporting synthetic and characterization work. D.P.M. supervised the synthetic component. M.J.G., M.V. and N.F.C. collected and interpreted EPR data. M.V. and N.F.C. performed CASSCF calculations. N.F.C. supervised the EPR and CASSCF components. S.M.G. collected and interpreted Mössbauer spectra and performed DFT calculations. S.H. supervised S.M.G. and provided additional EPR/Mössbauer interpretation. D.P.M. and N.F.C. wrote the manuscript, with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–80, Discussion and Tables 1–20.
Rights and permissions
About this article
Cite this article
Goodwin, C.A.P., Giansiracusa, M.J., Greer, S.M. et al. Isolation and electronic structures of derivatized manganocene, ferrocene and cobaltocene anions. Nat. Chem. 13, 243–248 (2021). https://doi.org/10.1038/s41557-020-00595-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00595-w
This article is cited by
-
Synthesis and characterization of a formal 21-electron cobaltocene derivative
Nature Communications (2023)