Abstract
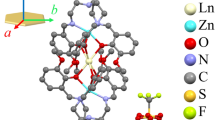
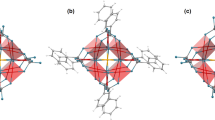
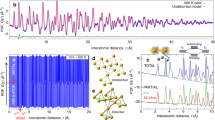
The distribution of electrons in the 4f orbitals of lanthanide ions is often assigned a crucial role in the design of single-molecule magnets, which maintain magnetization in zero external field. Optimal spatial complementarity between the 4f-electron density and the ligand field is key to maximizing magnetic anisotropy, which is an important factor in the ability of lanthanide complexes to display single-molecule magnet behaviour. Here we have experimentally determined the electron density distribution in two dysprosium molecular complexes by interpreting high-resolution synchrotron X-ray diffraction with a multipole model. The ground-state 4f-electron density is found to be an oblate ellipsoid, as is often deduced from a simplified Sievers model that assumes a pure |±15/2> ground-state doublet for the lanthanide ion. The large equatorial asymmetry—determined by a model wavefunction—was found to contain considerable MJ mixing of |±11/2> and only 81% of |±15/2>. The experimental molecular magnetic easy axes were recovered, and found to deviate by 13.1° and 8.7° from those obtained by ab initio calculations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition nos. CCDC 1900925 (1A) and 1900926 (1B). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings of this study are available in the Article and its Supplementary Information or from the corresponding author on reasonable request.
References
Bottrill, M., Kwok, L. & Long, N. J. Lanthanides in magnetic resonance imaging. Chem. Soc. Rev. 35, 557–571 (2006).
Otting, G. Protein NMR using paramagnetic ions. Ann. Rev. Biophys. 39, 387–405 (2010).
Leuenberger, M. N. & Loss, D. Quantum computing in molecular magnets. Nature 410, 789–793 (2001).
Troiani, F. et al. Molecular engineering of antiferromagnetic rings for quantum computation. Phys. Rev. Lett. 94, 207208 (2005).
Godfrin, C. et al. Operating quantum states in single magnetic molecules: implementation of Grover’s quantum algorithm. Phys. Rev. Lett. 119, 187702 (2017).
Sanvito, S. Molecular spintronics. Chem. Soc. Rev. 40, 3336–3355 (2011).
Lis, T. Preparation, structure, and magnetic properties of a dodecanuclear mixed-valence manganese carboxylate. Acta Crystallogr. B 36, 2042–2046 (1980).
Sessoli, R., Gatteschi, D., Caneschi, A. & Novak, M. A. Magnetic bistability in a metal–ion cluster. Nature 365, 141–143 (1993).
Sessoli, R. & Powell, A. K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 253, 2328–2341 (2009).
Blagg, R. J., Muryn, C. A., McInnes, E. J. L., Tuna, F. & Winpenny, R. E. P. Single pyramid magnets: Dy-5 pyramids with slow magnetic relaxation to 40 K. Angew. Chem. Int. Ed. 50, 6530–6533 (2011).
Samuel, P. P. et al. Electronic structure and slow magnetic relaxation of low-coordinate cyclic alkyl(amino) carbene stabilized iron(i) complexes. J. Am. Chem. Soc. 136, 11964–11971 (2014).
Mallah, T. et al. Magnetic anisotropy in pentacoordinate Ni(ii) and Co(ii) complexes: unraveling electronic and geometrical contributions. Chem. Eur. J. 23, 3648–3657 (2017).
Rinehart, J. D., Fang, M., Evans, W. J. & Long, J. R. A N2 3– radical-bridged terbium complex exhibiting magnetic hysteresis at 14 K. J. Am. Chem. Soc. 133, 14236–14239 (2011).
Craig, G. A. & Murrie, M. 3D single-ion magnets. Chem. Soc. Rev. 44, 2135–2147 (2015).
Bar, A. K., Pichon, C. & Sutter, J.-P. Magnetic anisotropy in two- to eight-coordinated transition–metal complexes: recent developments in molecular magnetism. Coord. Chem. Rev. 308, 346–380 (2016).
Rinehart, J. D. & Long, J. R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2, 2078–2085 (2011).
Gatteschi, D., Sessoli, R. & Villain, J. Molecular Nanomagnets (Oxford Univ. Press, 2006).
Sievers, J. Asphericity of 4f-shells in their hund rule ground-states. Z. Phys. B 45, 289–296 (1982).
Chilton, N. F., Collison, D., McInnes, E. J., Winpenny, R. E. & Soncini, A. An electrostatic model for the determination of magnetic anisotropy in dysprosium complexes. Nat. Commun. 4, 2551 (2013).
Goodwin, C. A. P., Ortu, F., Reta, D., Chilton, N. F. & Mills, D. P. Molecular magnetic hysteresis at 60 Kelvin in dysprosocenium. Nature 548, 439–442 (2017).
Chilton, N. F., Goodwin, C. A. P., Mills, D. P. & Winpenny, R. E. P. The first near-linear bis(amide) f-block complex: a blueprint for a high temperature single molecule magnet. Chem. Commun. 51, 101–103 (2015).
Gupta, S. K., Rajeshkumar, T., Rajaraman, G. & Murugavel, R. An air-stable Dy(iii) single-ion magnet with high anisotropy barrier and blocking temperature. Chem. Sci. 7, 5181–5191 (2016).
Meng, Y.-S. et al. Low-coordinate single-ion magnets by intercalation of lanthanides into a phenol matrix. Angew. Chem. Int. Ed. 57, 4673–4676 (2018).
Guo, F.-S. et al. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 362, 1400–1403 (2018).
Koritsanszky, T. S. & Coppens, P. Chemical applications of X-ray charge-density analysis. Chem Rev 101, 1583–1627 (2001).
Ananyev, I. V., Nelyubina, Y. V., Puntus, L. N., Lyssenko, K. A. & Eremenko, I. L. Peculiarities of metal—ligand bonding in europium trinitrate complexes: a viewpoint of comparative charge density analysis in crystals. Russian Chem. Bull. 65, 1178–1188 (2016).
Puntus, L. N., Lyssenko, K. A., Antipin, M. Y. & Bünzli, J.-C. G. Role of inner- and outer-sphere bonding in the sensitization of EuIII-luminescence deciphered by combined analysis of experimental electron density distribution function and photophysical data. Inorg. Chem. 47, 11095–11107 (2008).
Schmokel, M. S. et al. Comparative study of X-ray charge-density data on CoSb3. Acta Crystallogr. A 69, 570–582 (2013).
Schmokel, M. S. et al. Testing the concept of hypervalency: charge density analysis of K2SO4. Inorg. Chem. 51, 8607–8616 (2012).
Clausen, H. F. et al. Intermolecular interactions and electrostatic properties of the beta-hydroquinone apohost: implications for supramolecular chemistry. J. Phys. Chem. A 115, 12962–12972 (2011).
Coppens, P., Iversen, B. & Larsen, F. K. The use of synchrotron radiation in X-ray charge density analysis of coordination complexes. Coord. Chem. Rev. 249, 179–195 (2005).
Iversen, B. B. et al. Accurate charge densities in days—use of synchrotrons, image plates and very low temperatures. Acta Crystallogr. B 55, 363–374 (1999).
Dong, Y., Yan, P., Zou, X. & Li, G. Azacyclo-auxiliary ligand-tuned SMMs of dibenzoylmethane Dy(iii) complexes. Inorg. Chem. Front. 2, 827–836 (2015).
Klahn, E. A. et al. Mapping the magnetic anisotropy at the atomic scale in dysprosium single-molecule magnets. Chem. Eur. J. 24, 16576–16581 (2018).
Blessing, R. H. Data reduction and error analysis for accurate single crystal diffraction intensities. Crystallogr. Rev. 1, 3–58 (1987).
Blessing, R. H. & Langs, D. A. Data averaging with normal down-weighting of outliers. J. Appl. Crystallogr. 20, 427–428 (1987).
Hansen, N. K. & Coppens, P. Testing aspherical atom refinements on small-molecule data sets. Acta Crystallogr. Sect. A 34, 909–921 (1978).
Sheldrick, G. SHELXT—integrated space-group and crystal-structure determination. Acta Crystallogr. A 71, 3–8 (2015).
Sheldrick, G. M. A short history of SHELX. Acta Crystallogr. Sect. A 64, 112–122 (2008).
Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 42, 339–341 (2009).
Volkov, A. et al. XD2006 (Univ. Glasgow, 2006).
Petříček, V., Dušek, M. & Palatinus, L. Crystallographic computing system JANA2006: general features. Z. Kristallogr. Cryst. Mater. 229, 345 (2014).
Aquilante, F. et al. Molcas 8: new capabilities for multiconfigurational quantum chemical calculations across the periodic table. J Comput. Chem. 37, 506–541 (2016).
Chibotaru, L. F. & Ungur, L. Ab initio calculation of anisotropic magnetic properties of complexes. I. Unique definition of pseudospin Hamiltonians and their derivation. J. Chem. Phys. 137, 064112 (2012).
Genoni, A. X-ray constrained extremely localized molecular orbitals: theory and critical assessment of the new technique. J. Chem. Theory Comput. 9, 3004–3019 (2013).
Obara, S. & Saika, A. Efficient recursive computation of molecular integrals over Cartesian Gaussian functions. J. Chem. Phys. 84, 3963–3974 (1986).
Head-Gordon, M. & Pople, J. A. A method for two-electron Gaussian integral and integral derivative evaluation using recurrence relations. J. Chem. Phys. 89, 5777–5786 (1988).
Tellgren, E. I., Soncini, A. & Helgaker, T. Nonperturbative calculations in strong magnetic fields using London orbitals. J. Chem. Phys. 129, 154114 (2008).
Acknowledgements
The authors are grateful to V. Petricek for discussions about multipole modelling of lanthanide compounds using Jana2006. M. Sist, V. Hathwar, H. Kasai and K. Sugimoto are thanked for their help during synchrotron data collection. A.S. acknowledges support from the Australian Research Council (Future Fellowship no. FT180100519). J.O. acknowledges the financial support from Independent Research Foundation Denmark, the Danish National Research Foundation (DNRF-93), VILLUM FOUNDATION and Danscatt. S.J. and S.G. appreciate the support of the National Natural Science Foundation of China (21621061, 21822301, 21601005) and the National Basic Research Program of China (2017YFA0204903, 2018YFA0306003). The synchrotron experiment was performed on beamline BL02B2 at SPring-8 with the approval of the Japan Synchrotron Radiation Research Institute as a Partner User (proposal no. 2016B0078).
Author information
Authors and Affiliations
Contributions
J.O. designed the study. C.G. analysed experimental data. A.S. performed the theoretical analysis. S.J. synthesized the crystals. A.G. calculated theoretical structure factors. J.O., A.S. and C.G. co-wrote the manuscript, with the help of the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Details about the diffraction data collection, reduction and analysis. Computational details and results of energy levels, spatial shape of the electron density and ellipsoid fitting of surfaces.
Crystallographic data
CIF for compound 1A (CCDC reference 1900925).
Crystallographic data
Structure factors for compound 1A (CCDC reference 1900925).
Crystallographic data
CIF for compound 1B (CCDC reference 1900926).
Crystallographic data
Structure factors for compound 1B (CCDC reference 1900926).
Rights and permissions
About this article
Cite this article
Gao, C., Genoni, A., Gao, S. et al. Observation of the asphericity of 4f-electron density and its relation to the magnetic anisotropy axis in single-molecule magnets. Nat. Chem. 12, 213–219 (2020). https://doi.org/10.1038/s41557-019-0387-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0387-6
This article is cited by
-
Approaching the uniaxiality of magnetic anisotropy in single-molecule magnets
Science China Chemistry (2023)
-
The periodic table and the physics that drives it
Nature Reviews Chemistry (2020)