Abstract
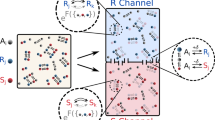
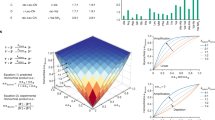
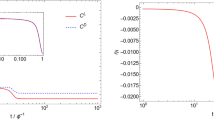
The homochirality of biological molecules (right-handed sugars and left-handed amino acids) is a signature of life. Extensive research has been devoted to understanding how enrichment of one enantiomer over the other might have emerged from a prebiotic world. Here, we use experimental data from the model Soai autocatalytic reaction system to evaluate the energy required for symmetry breaking and chiral amplification in molecular self-replication. One postulate for the source of the original imbalance is the tiny difference in energy between enantiomers due to parity violation in the weak force. We discuss the plausibility of parity violation energy difference coupled with asymmetric autocatalysis as a rationalization for absolute asymmetric synthesis and the origin of the homochirality of biological molecules. Our results allow us to identify the magnitude of the energy imbalance that gives rise to directed symmetry breaking and asymmetric amplification in this autocatalytic system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data supporting the findings of this study are available within the paper and its Supplementary Information files and/or are available on request from the authors.
Code availability
The Mathematica code used in this stochastic modelling is available on request from the authors.
References
Lee, T. D. & Yang, C. N. Question of parity conservation in weak interactions. Phys. Rev. 104, 254–258 (1956).
Wu, C. S., Ambler, E., Hayward, R. W., Hoppes, D. & Hudson, R. Experimental test of parity conservation in beta decay. Phys. Rev. 105, 1413–1415 (1957).
Garwin, R. L., Lederman, L. M. & Weinrich, M. Observations of the failure of conservation of parity and charge conjugation in meson decays: the magnetic moment of the free muon. Phys. Rev. 105, 1415–1417 (1957).
Yamagata, Y. A hypothesis for the asymmetric appearance of biomolecules on Earth. J. Theor. Biol. 11, 495–498 (1966).
Quack, M. How important is parity violation for molecular and biomolecular chirality? Angew. Chem. Int. Ed. 41, 4618–4630 (2002).
Frank, F. C. On spontaneous asymmetric synthesis. Biochim. Biophys. Acta 11, 459–463 (1953).
Soai, K., Shibata, T., Morioka, H. & Choji, K. Asymmetric autocatalysis and amplification of enantiomeric excess of a chiral molecule. Nature 378, 767–768 (1995).
Blackmond, D. G., McMillan, C. R., Ramdeehul, S., Schorm, A. & Brown, J. M. Origins of asymmetric amplification in autocatalytic alkylzinc additions. J. Am. Chem. Soc. 123, 10103–10104 (2001).
Blackmond, D. G. Description of the condition for asymmetric amplification in autocatalytic reactions. Adv. Synth. Catal. 344, 156–158 (2002).
Blackmond, D. G. Asymmetric autocatalysis and its implications for the origin of homochirality. Proc. Natl Acad. Sci. USA 101, 5732–5736 (2004).
Gridnev, I. D., Serafimov, J. M. & Brown, J. M. Solution structure and reagent binding of the zinc alkoxide catalyst in the Soai asymmetric autocatalytic reaction. Angew. Chem. Int. Ed. 43, 4884–4887 (2004).
Jafarpour, F., Biancalani, T. & Goldenfeld, N. Noise-induced mechanism for biological homochirality of early life self-replicators. Phys. Rev. Lett. 115, 158101 (2015).
Stich, M., Ribó, J. M., Blackmond, D. G. & Hochberg, D. Necessary conditions for the emergence of homochirality via autocatalytic self-replication. J. Chem. Phys. 145, 074111 (2016).
Blackmond, D. G. Mechanistic study of the Soai reaction informed by kinetic analysis. Tetrahedron Asymm. 17, 584–589 (2006).
Buono, F. G. & Blackmond, D. G. Kinetic evidence for a tetrameric transition state in the asymmetric alkylation of pyrimidyl aldehydes. J. Am. Chem. Soc. 125, 8978–8979 (2003).
Gridnev, I. D., Serafimov, J. M., Quiney, H. & Brown, J. M. Reflections on spontaneous asymmetric synthesis by amplifying autocatalysis. Org. Biomol. Chem. 1, 3811–3819 (2003).
Quaranta, M., Gehring, T., Odell, B., Brown, J. M. & Blackmond, D. G. Unusual inverse temperature dependence on reaction rate in the asymmetric autocatalytic alkylation of pyrimidyl aldehydes. J. Am. Chem. Soc. 132, 15104–15107 (2010).
Gridnev, I. D. & Vorobiev, A. K. Quantification of sophisticated equilibria in the reaction pool and amplifying catalytic cycle of the Soai reaction. ACS Catal. 2, 2137–2149 (2012).
Matsumoto, A. et al. Crystal structure of the isopropylzinc alkoxide of pyrimidyl alkanol: mechanistic insights for asymmetric autocatalysis with amplification of enantiomeric excess. Angew. Chem. Int. Ed. 54, 15218–15221 (2015).
Schiaffino, L. & Ercolani, E. Amplification of chirality and enantioselectivity in the asymmetric autocatalytic Soai reaction. ChemPhysChem 10, 2508–2515 (2009).
Shibata, T. et al. Amplification of a slight enantiomeric imbalance in molecules based on asymmetric autocatalysis: the first correlation between high enantiomeric enrichment in a chiral molecule and circularly polarized light. J. Am. Chem. Soc. 120, 12157–12158 (1998).
Soai, K. et al. d- and l-Quartz-promoted highly enantioselective synthesis of a chiral organic compound. J. Am. Chem. Soc. 121, 11235–11236 (1999).
Kawasaki, T. et al. Asymmetric autocatalysis triggered by carbon isotope (13C/12C). Science 324, 492–495 (2009).
Kawasaki, T. et al. Asymmetric autocatalysis induced by meteoritic amino acids with hydrogen isotope chirality. Chem. Commun. 2009, 4396–4398 (2009).
Kawasaki, T. et al. Asymmetric autocatalysis triggered by chiral isotopomer arising from oxygen isotope substitution. Angew. Chem. Int. Ed. 50, 8131–8133 (2011).
Matsumoto, A. et al. Asymmetric induction by a nitrogen 14N/15N isotopomer in conjunction with asymmetric autocatalysis. Angew. Chem. Int. Ed. 55, 15246–15249 (2016).
Sato, I., Omiya, D., Saito, T. & Soai, K. Highly enantioselective synthesis induced by chiral primary alcohols due to deuterium substitution. J. Am. Chem. Soc. 122, 11739–11740 (2000).
Hawbaker, N. A. & Blackmond, D. G. Rationalization of asymmetric amplification via autocatalysis triggered by isotopically chiral molecules. ACS Central Sci. 4, 776–780 (2018).
Buono, F. G., Iwamura, H. & Blackmond, D. G. Physical and chemical rationalization for asymmetric amplification in autocatalytic reactions. Angew. Chem. Int. Ed. 43, 2099–2103 (2004).
Saiki, R. et al. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science 230, 1350–1354 (1985).
Schnoerr, D., Sanguinetti, G. & Grima, R. Approximation and inference methods for stochastic biochemical kinetics—a tutorial review. J. Phys. A Math. Theor. 50, 093001 (2017).
Kondepudi, D. K. & Asakura, A. Chiral autocatalysis, spontaneous symmetry breaking, and stochastic behavior. Acc. Chem. Res. 34, 946–954 (2001).
Kondepudi, D. K. & Nelson, G. W. Weak neutral currents and the origin of biomolecular chirality. Nature 314, 438–441 (1985).
Kondepudi, D. K. & Nelson, G. W. Chiral symmetry breaking in non-equilibrium systems. Phys. Rev. Lett. 50, 1023–1026 (1983).
Sato, I., Urabe, H., Ishiguro, S., Shibata, T. & Soai, K. Amplification of chirality from extremely low to greater than 99.5% ee by asymmetric autocatalysis. Angew. Chem. Int. Ed. 42, 315–317 (2003).
Singleton, D. A. & Vo, L. K. Enantioselective synthesis without discrete optically active additives. J. Am. Chem. Soc. 124, 10010–10011 (2002).
Singleton, D. A. & Vo, L. K. A few molecules can control the enantiomeric outcome. Evidence supporting absolute asymmetric synthesis using the Soai asymmetric autocatalysis. Org. Lett. 5, 4337–4339 (2003).
Maioli, M., Varadi, G., Kurdi, R., Caglioti, L. & Pályi, G. Limits of the classical concept of concentration. J. Phys. Chem. B 120, 7438–7445 (2016).
Lente, G. The effect of parity violation on kinetic models of enantioselective autocatalysis. Phys. Chem. Chem. Phys. 9, 6134–6141 (2007).
Quack, M. On biomolecular homochirality as a quasi-fossil of the evolution of life. Adv. Chem. Phys. 157, 249–290 (2015).
Berger, R. & Quack, M. Electroweak quantum chemistry of alanine: parity violation in gas and condensed phases. ChemPhysChem 1, 57–60 (2000).
Von Kiedrowski, G. A self-replicating hexadeoxynucleotide. Angew. Chem. Int. Ed. 25, 932–935 (1986).
Plöger, T. A. & von Kiedrowski, G. A self-replicating peptide nucleic acid. Org. Biomol. Chem. 12, 6908–6914 (2014).
Bag, B. G. & von Kiedrowski, G. Templates, autocatalysis, and molecular replication. Pure Appl. Chem. 68, 2145–2152 (1996).
Orgel, L. E. The implausibility of metabolic cycles on the prebiotic Earth. PLoS Biol. 6, 0005–0013 (2008).
Blackmond, D. G. An examination of the role of autocatalytic cycles in the chemistry of proposed primordial reactions. Angew. Chem. Int. Ed. 48, 386–390 (2009).
Budin, I. & Szostak, J. W. Expanding roles for diverse physical phenomena during the origin of life. Annu. Rev. Biophys. 39, 245–263 (2010).
Acknowledgements
D.G.B. acknowledges funding from the Simons Foundation through the Simons Collaboration on the Origins of Life (SCOL 287625). N.A.H. acknowledges a US Department of Defense SMART (Science, Mathematics, and Research for Transformation) Scholarship for Service. We are grateful to D. K. Kondepudi for stimulating discussions and guidance in stochastic modelling. We also acknowledge helpful discussions with J. M. Brown, G. F. Joyce and S. E. Denmark.
Author information
Authors and Affiliations
Contributions
N.A.H. carried out the experimental and modelling studies and provided input in the writing. D.G.B. conceived the project, supervised the experimental work and interpretation of the experimental and modelling studies, and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary information providing details of the experimental and computational methods, full tables of all experimental symmetry-breaking experiments, and figures showing the results of kinetic modelling.
Rights and permissions
About this article
Cite this article
Hawbaker, N.A., Blackmond, D.G. Energy threshold for chiral symmetry breaking in molecular self-replication. Nat. Chem. 11, 957–962 (2019). https://doi.org/10.1038/s41557-019-0321-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0321-y
This article is cited by
-
Uncovering the chiral bias of meteoritic isovaline through asymmetric photochemistry
Nature Communications (2023)
-
Chiral recognition of neutral guests by chiral naphthotubes with a bis-thiourea endo-functionalized cavity
Nature Communications (2023)
-
Parity Violation Energy Difference Calculation of Atropisomers
Origins of Life and Evolution of Biospheres (2023)
-
Evolutionary Approach to Biological Homochirality
Origins of Life and Evolution of Biospheres (2022)
-
Magnetic circular dichroism in Archean atmosphere and asymmetric photolysis of biomolecules: enantiomeric excess of prebiotic sugar
Journal of Biological Physics (2020)