Abstract
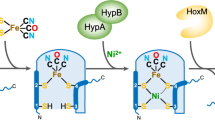
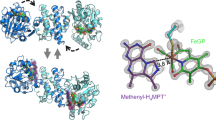
Nature carefully selects specific metal ions for incorporation into the enzymes that catalyse the chemical reactions necessary for life. Hydrogenases, enzymes that activate molecular H2, exclusively utilize Ni and Fe in [NiFe]-, [FeFe]- and [Fe]-hydrogeanses. However, other transition metals are known to activate or catalyse the production of hydrogen in synthetic systems. Here, we report the development of a biomimetic model complex of [Fe]-hydrogenase that incorporates a Mn, as opposed to a Fe, metal centre. This Mn complex is able to heterolytically cleave H2 as well as catalyse hydrogenation reactions. The incorporation of the model into an apoenzyme of [Fe]-hydrogenase results in a [Mn]-hydrogenase with an enhanced occupancy-normalized activity over an analogous semi-synthetic [Fe]-hydrogenase. These findings demonstrate a non-native metal hydrogenase that shows catalytic functionality and that hydrogenases based on a manganese active site are viable.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available from the corresponding authors upon reasonable request. CCDC-1856616 and CCDC-1856615 contain the supplementary crystallographic data for 3 and 4(18-crown-6), respectively. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
References
Coleman, J. E. Metal ion dependent binding of sulphonamide to carbonic anhydrase. Nature 214, 193–194 (1967).
Cuatrecasas, P., Fuchs, S. & Anfinsen, C. B. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J. Biol. Chem. 242, 1541–1547 (1967).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Lubitz, W., Ogata, H., Rudiger, O. & Reijerse, E. Hydrogenases. Chem. Rev. 114, 4081–4148 (2014).
Sommer, C., Richers, C. P., Lubitz, W., Rauchfuss, T. B. & Reijerse, E. J. A. [RuRu] analogue of an [FeFe]-hydrogenase traps the key hydride intermediate of the catalytic cycle. Angew. Chem. Int. Ed. 57, 5429–5432 (2018).
Shima, S. & Ermler, U. Structure and function of [Fe]-hydrogenase and its iron-guanylylpyridinol (FeGP) cofactor. Eur. J. Inorg. Chem. 2011, 963–972 (2011).
Thauer, R. K., Kaster, A. K., Seedorf, H., Buckel, W. & Hedderich, R. Methanogenic archaea: ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 6, 579–591 (2008).
Shima, S. et al. The crystal structure of [Fe]-hydrogenase reveals the geometry of the active site. Science 321, 572–575 (2008).
Hiromoto, T. et al. The crystal structure of C176A mutated [Fe]-hydrogenase suggests an acyl-iron ligation in the active site iron complex. FEBS Lett. 583, 585–590 (2009).
Kallmeier, F. & Kempe, R. Manganese complexes for (de)hydrogenation catalysis: A comparison to cobalt and iron Catalysts. Angew. Chem. Int. Ed. 57, 46–60 (2018).
Xu, T. et al. A functional model of [Fe]-Hydrogenase. J. Am. Chem. Soc. 138, 3270–3273 (2016).
Shima, S. et al. Reconstitution of [Fe]-hydrogenase using model complexes. Nat. Chem. 7, 995–1002 (2015).
Wodrich, M. D. & Hu, X. Natural inspirations for metal–ligand cooperative catalysis. Nat. Rev. Chem. 2, 0099 (2017).
Zirngibl, C. et al. H-2-forming methylenetetrahydromethanopterin dehydrogenase, a novel type of hydrogenase without iron-sulfur clusters in methanogenic archaea. Eur. J. Biochem. 208, 511–520 (1992).
Klenk, H.-P. et al. The complete genome sequence of the hyperthermophilic, sulphate-reducing archaeon Archaeoglobus fulgidus. Nature 390, 364 (1997).
Acknowledgements
This work was supported by the Swiss National Science Foundation (to X.L.H.), European Union Marie Sklodowska-Curie Individual Fellowships (794000 to H.-J.P.), Max Planck Society (to S.S.) and Deutsche Forschungsgemeinschaft (SH 87/1-1, to S.S.). M.D.W. acknowledges C. Corminboeuf (EPFL)for financial support and the Laboratory for Computational Molecular Design (EPFL) for providing computing resources. G.H. was supported by a fellowship from the China Scholarship Council (CSC).
Author information
Authors and Affiliations
Contributions
S.S. and X.L.H. directed the research. H.-J.P. conducted the experiments of the Mn complexes; G.F.H. conducted experiments of the semi-synthetic enzymes; M.D.W. did the computations; F.F.T. did the crystallographic study of Mn complexes; K.A. performed infrared spectroscopy of the enzymes; All authors discussed the data. X.L.H. wrote the manuscript with contributions from H.-J.P and S.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Supplementary experimental procedures, experimental data, optimization data, compounds characterization data, and computational methods
Cif file for complex 3
Crystallographic file for complex 3; CCDC 1856616
Cif file for complex 4
Crystallographic file for complex 4(18-crown-6); CCDC 1856615
Rights and permissions
About this article
Cite this article
Pan, HJ., Huang, G., Wodrich, M.D. et al. A catalytically active [Mn]-hydrogenase incorporating a non-native metal cofactor. Nat. Chem. 11, 669–675 (2019). https://doi.org/10.1038/s41557-019-0266-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0266-1
This article is cited by
-
Biomimetic asymmetric catalysis
Science China Chemistry (2023)
-
Structure, reactivity and catalytic properties of manganese-hydride amidate complexes
Nature Chemistry (2022)
-
Simultaneous oxidative and reductive reactions in one system by atomic design
Nature Catalysis (2021)
-
Development of a practical non-noble metal catalyst for hydrogenation of N-heteroarenes
Nature Catalysis (2020)
-
Methanogenesis involves direct hydride transfer from H2 to an organic substrate
Nature Reviews Chemistry (2020)