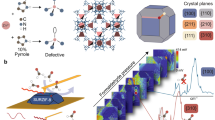
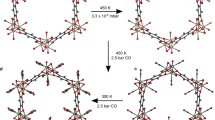
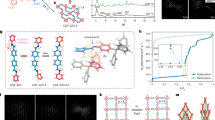
Abstract
Accurate measurements and assessments of gas adsorption isotherms are important to characterize porous materials and develop their applications. Although these isotherms provide knowledge of the overall gas uptake within a material, they do not directly give critical information concerning the adsorption behaviour of adsorbates in each individual pore, especially in porous materials in which multiple types of pore are present. Here we show how gas adsorption isotherms can be accurately decomposed into multiple sub-isotherms that correspond to each type of pore within a material. Specifically, two metal–organic frameworks, PCN-224 and ZIF-412, which contain two and three different types of pore, respectively, were used to generate isotherms of individual pores by combining gas adsorption measurements with in situ X-ray diffraction. This isotherm decomposition approach gives access to information about the gas uptake capacity, surface area and accessible pore volume of each individual pore, as well as the impact of pore geometry on the uptake and distribution of different adsorbates within the pores.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data that support the findings in this study are available within the article and its Supplementary Information and/or from the corresponding authors on reasonable request.
Code availability
The program VESTA was coded by K.M. and is available free of charge via public domain at http://jp-minerals.org/vesta/en/.
References
Allison, S.A. & Barrer, R.M. Sorption in the β -phases of transition metal(II) tetra-(4-methylpyridine)thiocyanates and related compounds. J. Chem. Soc. A Inorg. Phys. Theor. 0, 1717–1723 (1969).
Li, H., Eddaoudi, M., Gory, T. L. & Yaghi, O. M. Establishing microporosity in open metal–organic frameworks: gas adsorption isotherm for Zn(BDC) (BDC = 1,4-benzenedicarboxylate). J. Am. Chem. Soc. 120, 8571–8572 (1998).
Furukawa, H., Cordova, K. E., O’Keeffe, M. & Yaghi, O. M. The chemistry and applications of metal–organic frameworks. Science 341, 1230444 (2013).
Kitagawa, S., Kitaura, R. & Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 43, 2334–2375 (2004).
Rosi, N. L. et al. Hydrogen storage in microporous metal–organic frameworks. Science 300, 1127–1129 (2003).
Makal, T. A., Li, J., Lu, W. & Zhou, H. Methane storage in advanced porous materials. Chem. Soc. Rev. 41, 7761–7779 (2012).
Nugent, P. et al. Porous materials with optimal adsorption thermodynamics and kinetics for CO2 separation. Nature 495, 80–84 (2013).
Sumida, K. et al. Carbon dioxide capture in metal–organic frameworks. Chem. Rev. 112, 724–781 (2012).
Muroyama, N. et al. Argon adsorption on MCM-41 mesoporous crystal studied by in situ synchrotron powder X-ray diffraction. J. Phys. Chem. C 112, 10803–10813 (2008).
Jafta, C. J. et al. Correlating pore size and shape to local disorder in microporous carbon: a combined small angle neutron and X-ray scattering study. Carbon 123, 440–447 (2017).
Rowsell, J. L. C., Spencer, E. C., Eckert, J., Howard, J. A. K. & Yaghi, O. M. Gas adsorption sites in a large-pore metal–organic framework. Science 309, 1350–1354 (2005).
Yan, Y. et al. Metal−organic polyhedral frameworks: high H2 adsorption capacities and neutron powder diffraction studies. J. Am. Chem. Soc. 132, 4092–4094 (2010).
Olds, D. et al. Capturing the details of N2 adsorption in zeolite X using stroboscopic isotope contrasted neutron total scattering. Chem. Mater. 30, 296–302 (2018).
Yoshimoto, M. et al. Mesoscopic investigation of an ‘Immiscible’ cyclohexane and water micro-mixture in carbon micropores by contrast variation small-angle neutron scattering. Chem. Lett. 47, 336–339 (2018).
Giacobbe, C., Lavigna, E., Maspero, A. & Galli, S. Elucidating the CO2 adsorption mechanism in the triangular channels of the bis(pyrazolate) MOF Fe2 (BPEB)3 by in situ synchrotron X-ray diffraction and molecular dynamics simulations. J. Mater. Chem. A 5, 16964–16975 (2017).
Miller, S. R. et al. Single crystal X-ray diffraction studies of carbon dioxide and fuel-related gases adsorbed on the small pore scandium terephthalate metal organic framework, Sc2(O2CC6H4CO2)3. Langmuir 25, 3618–3626 (2009).
Li, L. et al. Efficient separation of ethylene from acetylene/ethylene mixtures by a flexible-robust metal–organic framework. J. Mater. Chem. A 5, 18984–18988 (2017).
Carrington, E. J., Vitórica-Yrezábal, I. J. & Brammer, L. Crystallographic studies of gas adsorption in metal–organic frameworks. Acta Cryst. B 70, 404–422 (2014).
Ghosh, S. K., Bureekaew, S. & Kitagawa, S. A dynamic, isocyanurate-functionalized porous coordination polymer. Angew. Chem. Int. Ed. 47, 3403–3406 (2008).
Krause, S. et al. A pressure-amplifying framework material with negative gas adsorption transitions. Nature 532, 348–352 (2016).
Cho, H. S. et al. Extra adsorption and adsorbate superlattice formation in metal–organic frameworks. Nature 527, 503–507 (2015).
Feng, D. et al. Construction of ultrastable porphyrin Zr metal–organic frameworks through linker elimination. J. Am. Chem. Soc. 135, 17105–17110 (2013).
Yang, J. et al. Principles of designing extra-large pore openings and pores in zeolitic imidazolate frameworks. J. Am. Chem. Soc. 139, 6448–6455 (2017).
Yuan, D., Zhao, D., Sun, D. & Zhou, H. An isoreticular series of metal–organic frameworks with dendritic hexacarboxylate ligands and exceptionally high gas-uptake capacity. Angew. Chem. Int. Ed. 49, 5357–5361 (2010).
Koh, K., Wong-Foy, A. G. & Matzger, A. J. A porous coordination copolymer with over 5000 m2/g BET surface area. J. Am. Chem. Soc. 131, 4184–4185 (2009).
Farha, O. K. et al. De novo synthesis of a metal–organic framework material featuring ultrahigh surface area and gas storage capacities. Nat. Chem. 2, 944–948 (2010).
Liu, H. et al. A porous zirconium-based metal–organic framework with the potential for the separation of butene isomers. Chem. Eur. J. 22, 14988–14997 (2016).
Li, P. et al. Toward design rules for enzyme immobilization in hierarchical mesoporous metal–organic frameworks. Chem 1, 154–169 (2016).
Liang, C. et al. Engineering of pore geometry for ultrahigh capacity methane storage in mesoporous metal–organic frameworks. J. Am. Chem. Soc. 139, 13300–13303 (2017).
Thommes, M. et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 87, 1051–1069 (2015).
Collins, D. M. Electron density images from imperfect data by iterative entropy maximization. Nature 298, 49–51 (1982).
Momma, K., Ikeda, T., Belik, A. A. & Izumi, F. Dysnomia, a computer program for maximum-entropy method (MEM) analysis and its performance in the MEM-based pattern fitting. Powder Diffr. 28, 184–193 (2013).
Lastoskie, C., Gubbins, K. E. & Quirke, N. Pore size distribution analysis of microporous carbons: a density functional theory approach. J. Phys. Chem. 97, 4786–4796 (1993).
Ravikovitch, P. I. & Neimark, A. V. Density functional theory model of adsorption on amorphous and microporous silica materials. Langmuir 22, 11171–11179 (2006).
Evans, R., Marconi, U. M. B. & Tarazona, P. Capillary condensation and adsorption in cylindrical and slit-like pores. J. Chem. Soc. Faraday Trans. II 82, 1763–1787 (1986).
Rouquerol, J. et al. Recommendations for the characterization of porous solids. Pure Appl. Chem. 66, 1739–1758 (1994).
Brunauer, S., Emmett, P. H. & Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 60, 309–319 (1938).
Barrett, E. P., Joyner, L. G. & Halenda, P. P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 73, 373–380 (1951).
Walton, K. S. & Snurr, R. Q. Applicability of the BET method for determining surface areas of microporous metal−organic frameworks. J. Am. Chem. Soc. 129, 8552–8556 (2007).
Deng, H. et al. Large-pore pore openings in a series of metal–organic frameworks. Science 336, 1018–1023 (2012).
Peng, Y. et al. Methane storage in metal–organic frameworks: current records, surprise findings, and challenges. J. Am. Chem. Soc. 135, 11887–11894 (2013).
Gandara, F., Furukawa, H., Lee, S. & Yaghi, O. M. High methane storage capacity in aluminum metal–organic frameworks. J. Am. Chem. Soc. 136, 5271–5274 (2014).
Mason, J. A. et al. Methane storage in flexible metal–organic frameworks with intrinsic thermal management. Nature 527, 357–361 (2015).
Alezi, D. et al. MOF crystal chemistry paving the way to gas storage needs: aluminum-based soc-MOF for CH4, O2, and CO2 storage. J. Am. Chem. Soc. 137, 13308–13318 (2015).
Rouquerol, F., Rouquerol, J. & Sing, K. Adsorption by Powders and Porous Solid: Principle, Methodology, and Applications (Academic, San Diego, 1999).
Qajar, A., Daigle, H. & Prodinovic, M. The effect of pore geometry on adsorption equilibrium in shale formations and coal-beds: lattice density functional theory study. Fuel 163, 205–213 (2016).
Marra, G. L. et al. Cation location in dehydrated Na−Rb−Y zeolite: an XRD and IR study. J. Phys. Chem. B 101, 10653–10660 (1997).
Petříček, V., Dušek, M. & Palatinus, L. Crystallographic computing system JANA2006: general features. Z. Kristallogr. 229, 345–352 (2014).
Momma, K. & Izumi, K. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgements
We acknowledge financial support from BK21+ program, the Center for Hybrid Interface Materials (2013M3A6B1078884) and the National Research Foundation of Korea (2017M2A2A6A01070673) (H.S.C., J.K.K. and O.T.), CℏEM, SPST of ShanghaiTech University (no. EM02161943) (H.S.C. and O.T.), Foreign 1000 Talents Plan, China (O.T.), King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia (O.M.Y.), National Natural Science Foundation of China (no. 21471118, 91545205 and 91622103) and National Key Basic Research Program of China (no. 2014CB239203) (X.G. and H.D.), NSFC 21522105 (Y.B.Z.) and an Advanced European Research Grant (ERC, no. 321140) (H.S.C. and B.M.W.). We also thank R. Flaig for proofreading the manuscript, and X. Cai for providing the three-dimensional structure illustration of each individual cage.
Author information
Authors and Affiliations
Contributions
O.T. and O.M.Y. conceived the idea. O.T., O.M.Y. and H.D. led the project. H.S.C. performed the in situ XRD experiments and analysis. J.Y., X.G., Y.-B.Z. and H.D. prepared the samples, and K.M. coded the computer program VESTA. O.T, H.S.C. and J.K.K. contributed to set up the experimental system. H.S.C., J.Y., X.G., H.D., O.M.Y. and O.T. prepared the first version of the manuscript and all the authors contributed to the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary data, discussion and methods, Supplementary Figs. 1–52 and Supplementary references 1–11.
Rights and permissions
About this article
Cite this article
Cho, H.S., Yang, J., Gong, X. et al. Isotherms of individual pores by gas adsorption crystallography. Nat. Chem. 11, 562–570 (2019). https://doi.org/10.1038/s41557-019-0257-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0257-2
This article is cited by
-
Double-walled Al-based MOF with large microporous specific surface area for trace benzene adsorption
Nature Communications (2024)
-
Application of carbon materials in catalytic systems for the hydrogenation—dehydrogenation of liquid organic hydrogen carriers
Russian Chemical Bulletin (2024)
-
A spin-crossover framework endowed with pore-adjustable behavior by slow structural dynamics
Nature Communications (2022)
-
Rapid room-temperature synthesis of a porphyrinic MOF for encapsulating metal nanoparticles
Nano Research (2021)
-
Influence of the porous structure and functionality of the MIL type metal-organic frameworks and carbon matrices on the adsorption of 2,4-dichlorophenoxyacetic acid
Russian Chemical Bulletin (2021)