Abstract
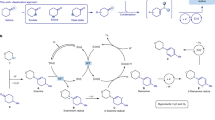
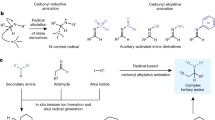
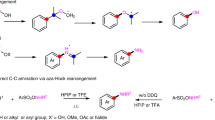
The formation of carbon–nitrogen bonds for the preparation of aromatic amines is among the top five reactions carried out globally for the production of high-value materials, ranging from from bulk chemicals to pharmaceuticals and polymers. As a result of this ubiquity and diversity, methods for their preparation impact the full spectrum of chemical syntheses in academia and industry. In general, these molecules are assembled through the stepwise introduction of a reactivity handle in place of an aromatic C–H bond (that is, a nitro group, halogen or boronic acid) and a subsequent functionalization or cross-coupling. Here we show that aromatic amines can be constructed by direct reaction of arenes and alkyl amines using photocatalysis, without the need for pre-functionalization. The process enables the easy preparation of advanced building blocks, tolerates a broad range of functionalities, and multigram scale can be achieved via a batch-to-flow protocol. The merit of this strategy as a late-stage functionalization platform has been demonstrated by the modification of several drugs, agrochemicals, peptides, chiral catalysts, polymers and organometallic complexes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Supplementary Information. These include reaction procedures, products characterization, the batch-to-flow experiment procedure, the microscale parallel screening procedure, cyclic voltammograms and UV–vis, density functional theory and NMR spectra.
References
Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences (Wiley, Hoboken, 2008).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Roughley, S. D. & Jordan, A. M. The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem. 54, 3451–3479 (2011).
Ruiz-Castillo, P. & Buchwald, S. L. Applications of palladium-catalyzed C−N cross-coupling reactions. Chem. Rev. 116, 12564–12649 (2016).
Hartwig, J. F. Evolution of a fourth generation catalyst for the amination and thioetherification of aryl halides. Acc. Chem. Res. 41, 1534–1544 (2008).
Corcoran, E. B. et al. Aryl amination using ligand-free Ni(ii) salts and photoredox catalysis. Science 353, 279–283 (2016).
Creutz, S. E., Lotito, K. J., Fu, G. C., Peters, J. C. & Ullmann, C. –N. Photoinduced coupling: demonstrating the viability of a radical pathway. Science 338, 647–651 (2012).
Santanilla, A. B. et al. Nanomole-scale high-throughput chemistry for the synthesis of complex molecules. Science 347, 44–49 (2015).
Jiao, J., Murakami, K. & Itami, K. Catalytic methods for aromatic C–H amination: an ideal strategy for nitrogen-based functional molecules. ACS Catal. 6, 610–633 (2016).
Allen, L. J., Cabrera, P. J., Lee, M. & Sanford, M. S. N-Acyloxyphthalimides as nitrogen radical precursors in the visible light photocatalyzed room temperature C–H amination of arenes and heteroarenes. J. Am. Chem. Soc. 136, 5607–5610 (2014).
Foo, K., Sella, E., Thomé, I., Eastgate, M. D. & Baran, P. S. A mild, ferrocene-catalyzed C–H imidation of (hetero)arenes. J. Am. Chem. Soc. 136, 5279–5282 (2014).
Boursalian, G. B., Ham, W. S., Mazzotti, A. R. & Ritter, T. Charge-transfer-directed radical substitution enables para-selective C–H functionalization. Nat. Chem. 8, 810–815 (2016).
Romero, N. A., Margrey, K. A., Tay, N. E. & Nicewicz, D. A. Site-selective arene C-H amination via photoredox catalysis. Science 349, 1326–1330 (2015).
Morofuji, T., Shimizu, A. & Yoshida, J. Direct C–N coupling of imidazoles with aromatic and benzylic compounds via electrooxidative C–H functionalization. J. Am. Chem. Soc. 136, 4496–4499 (2014).
Paudyal, M. P. et al. Dirhodium-catalyzed C-H arene amination using hydroxylamines. Science 353, 1144–1147 (2016).
Legnani, L., Cerai, G. P. & Morandi, B. Direct and practical synthesis of primary anilines through iron-catalyzed C−H bond amination. ACS Catal. 6, 8162–8165 (2016).
An, X.-D. & Yu, S. Photoredox-catalyzed C(sp 2)–N coupling reactions. Tetrahedron Lett. 59, 1605 (2018).
Chow, Y. L., Danen, W. C., Nelsen, S. F. & Rosenblatt, D. H. Nonaromatic aminium radicals. Chem. Rev. 78, 243–274 (1978).
Svejstrup, T. D., Ruffoni, A., Julia, F., Aubert, V. M. & Leonori, D. Synthesis of arylamines via aminium radicals. Angew. Chem. Int. Ed. 56, 14948–14952 (2017).
Margrey, K. A., Levens, A. & Nicewicz, D. A. Direct aryl C–H amination with primary amines using organic photoredox catalysis. Angew. Chem. Int. Ed. 56, 15644–15648 (2017).
Goldberg, F. W., Kettle, J. G., Kogej, T., Perry, M. W. D. & Tomkinson, N. P. Designing novel building blocks is an overlooked strategy to improve compound quality. Drug Discov. Today 20, 11–17 (2015).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Lee, S. J., Terrazas, M. S., Pippel, D. J. & Beak, P. Mechanism of electrophilic chlorination: experimental determination of a geometrical requirement for chlorine transfer by the endocyclic restriction test. J. Am. Chem. Soc. 125, 7307–7312 (2003).
Xiong, X. & Yeung, Y.-Y. Highly ortho-selective chlorination of anilines using a secondary ammonium salt organocatalyst. Angew. Chem. Int. Ed. 55, 16101–16105 (2016).
Minisci, F. Novel applications of free-radical reactions in preparative organic chemistry. Synthesis 1973, 1–24 (1973).
Cosgrove, S. C., Plane, J. M. C. & Marsden, S. P. Radical-mediated direct C–H amination of arenes with secondary amines. Chem. Sci. 9, 6647–6652 (2018).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Musacchio, A. J. et al. Catalytic intermolecular hydroaminations of unactivated olefins with secondary alkyl amines. Science 355, 727 (2017).
Citterio, A. et al. Polar effects in fee radical reactions. homlytic aromatic amination by the amino radical cation, •+NH3: reactivity and selectivity. J. Org. Chem. 49, 4479–4482 (1984).
Colomer, I., Chamberlain, A. E. R., Haughey, M. B. & Donohoe, T. J. Hexafluoroisopropanol as a highly versatile solvent. Nat. Rev. Chem. 1, 0088 (2017).
Tang, R.-J., Milcent, T. & Crousse, B. Regioselective halogenation of arenes and heterocycles in hexafluoroisopropanol. J. Org. Chem. 83, 930–938 (2018).
Nguyen, J. D., Reiß, B., Dai, C. & Stephenson, C. R. J. Batch to flow deoxygenation using visible light photoredox catalysis. Chem. Commun. 49, 4352–4354 (2013).
Cambié, D., Bottecchia, C., Straathof, N. J. W., Hessel, V. & Noël, T. Applications of continuous-flow photochemistry in organic synthesis, material science, and water treatment. Chem. Rev. 116, 10276–10341 (2016).
Wang, H.-W. et al. Ligand-promoted rhodium(iii)-catalyzed ortho-C−H amination with free amines. Angew. Chem. Int. Ed. 56, 7449–7453 (2017).
Rosane, J. & Daugulis, O. A general method for aminoquinoline-directed, copper-catalyzed sp 2 C–H bond amination. J. Am. Chem. Soc. 138, 4601–4607 (2016).
Yoo, E. J., Ma, S., Mei, T.-S., Chan, K. S. L. & Yu, J.-Q. Pd-catalyzed Intermolecular C–H amination with alkylamines. J. Am. Chem. Soc. 133, 7652–7655 (2011).
Carreira, E. M. & Fessard, T. C. Four-membered ring-containing spirocycles: synthetic strategies and opportunities. Chem. Rev. 114, 8257–8322 (2014).
Willcox, D. et al. A general catalytic β-C–H carbonylation of aliphatic amines to β-lactams. Science 354, 851–857 (2016).
Wanka, L., Iqbal, K. & Schreiner, P. R. The lipophilic bullet hits the targets: medicinal chemistry of adamantane derivatives. Chem. Rev. 113, 3516–3604 (2013).
Immel, O. et al. Catalyst for the preparation of aniline. US patent 5,304,525A (1994).
Krska, S. W., DiRocco, D. A., Dreher, S. D. & Shevlin, M. The evolution of chemical high-throughput experimentation to address challenging problems in pharmaceutical synthesis. Acc. Chem. Res. 50, 2976–2985 (2017).
Gesmundo, N. J. et al. Nanoscale synthesis and affinity ranking. Nature 557, 228–232 (2018).
Vinogradova, E. V., Zhang, C., Spokoyny, A. M., Pentelute, B. L. & Buchwald, S. L. Organometallic palladium reagents for cysteine bioconjugation. Nature 526, 687–691 (2025).
Bloom, S. et al. Decarboxylative alkylation for site-selective bioconjugation of native proteins via oxidation potentials. Nat. Chem. 10, 205–211 (2018).
Osberger, T. J., Rogness, D. C., Kohrt, J. T., Stepan, A. F. & White, M. C. Oxidative diversification of amino acids and peptides by small-molecule iron catalysis. Nature 537, 214–219 (2016).
deGruyter, J. N., Malins, L. R. & Baran, P. S. Residue-specific peptide modification: a chemist’s guide. Biochemistry 56, 3863–3873 (2017).
Boutureira, O. & Bernardes, G. J. L. Advances in chemical protein modification. Chem. Rev. 115, 2174–2195 (2015).
Blasco, E., Sims, M. B., Goldmann, A. S., Sumerlin, B. S. & Barner-Kowollik, C. Polymer functionalization. Macromolecules 50, 5215–5252 (2017).
Bomben, P. G., Robson, K. C. D., Sedach, P. A. & Berlinguette, C. P. On the viability of cyclometalated Ru(ii) complexes for light-harvesting applications. Inorg. Chem. 48, 9631–9643 (2009).
Ma, D. L. et al. Antagonizing STAT3 dimerization with a rhodium(iii) complex. Angew. Chem. Int. Ed. 53, 9178–9182 (2014).
Gagliardo, M. et al. Organic transformations on σ-aryl organometallic complexes. Angew. Chem. Int. Ed. 46, 8558–8573 (2007).
Acknowledgements
The authors thank M. Simonetti and F. Juliá-Hernandez for useful discussions. D.L. thanks EPSRC for a Fellowship (EP/P004997/1) and the European Research Council for a research grant (758427). A.R. thanks the Marie Curie Actions for a Fellowship (703238).
Author information
Authors and Affiliations
Contributions
A.R., F.J. and D.L. designed the project. A.R., F.J., T.D.S. and A.J.M. performed all the synthetic experiments. J.J.D. performed the batch-to-flow optimization and scale-up. All authors analysed the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Synthetic procedures; products characterization; electrochemical, UV–vis, emission quenching and DFT studies; NMR spectra.
Rights and permissions
About this article
Cite this article
Ruffoni, A., Juliá, F., Svejstrup, T.D. et al. Practical and regioselective amination of arenes using alkyl amines. Nat. Chem. 11, 426–433 (2019). https://doi.org/10.1038/s41557-019-0254-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0254-5
This article is cited by
-
Metal free cross-dehydrogenative N-N coupling of primary amides with Lewis basic amines
Nature Communications (2024)
-
Site-selective arene C–H amination with iron-aminyl radical
Nature Catalysis (2024)
-
Pushing Boundaries: What’s Next in Metal-Free C–H Functionalization for Sulfenylation?
Topics in Current Chemistry (2024)
-
Transition-metal-free silylboronate-mediated cross-couplings of organic fluorides with amines
Nature Communications (2023)
-
High coenzyme affinity chimeric amine dehydrogenase based on domain engineering
Bioresources and Bioprocessing (2022)