Abstract
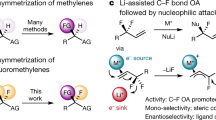
Given the unique properties of fluorine, and the ability of fluorination to change the properties of organic molecules, there is significant interest from medicinal chemists in innovative methodologies that enable the synthesis of new fluorinated motifs. State-of-the-art syntheses of α-fluorinated carbonyl compounds invariably rely on electrophilic fluorinating agents, which can be strongly oxidizing and difficult to handle. Here we show that reversing the polarity of the enolate partner to that of an enolonium enables nucleophilic fluorinating agents to be used for direct chemoselective α-C–H-fluorination of amides. Reduction of these products enables facile access to β-fluorinated amines and the value of this methodology is shown by the easy preparation of a number of fluorinated analogues of drugs and agrochemicals. A fluorinated analogue of citalopram, a marketed antidepressant drug, is presented as an example of the preserved biological activity after fluorination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Bondi, A. van der Waals volumes and radii. J. Phys. Chem. 68, 441–451 (1964).
O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 37, 308–319 (2008).
DeBernardis, J. F. et al. Conformationally defined adrenergic agents. 1. Design and synthesis of novel α2 selective adrenergic agents: electrostatic repulsion based conformational prototypes. J. Med. Chem. 28, 1398–1404 (1985).
Wang, J. et al. Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 114, 2432–2506 (2014).
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Purser, S., Moore, P. R., Swallow, S. & Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 37, 320–330 (2008).
Lankin, D. C., Chandrakumar, N. S., Rao, S. N., Spangler, D. P. & Snyder, J. P. Protonated 3-fluoropiperidines: an unusual fluoro directing effect and a test for quantitative theories of solvation. J. Am. Chem. Soc. 115, 3356–3357 (1993).
van Niel, M. B. et al. Fluorination of 3-(3-(piperidin-1-yl)propyl)indoles and 3-(3-(piperazin-1-yl)propyl)indoles gives selective human 5-HT1D receptor ligands with improved pharmacokinetic profiles. J. Med. Chem. 42, 2087–2104 (1999).
Park, B. K., Kitteringham, N. R. & O’Neill, P. M. Metabolism of fluorine-containing drugs. Annu. Rev. Pharmacol. Toxicol. 41, 443–470 (2001).
Banks, R. E., & Mohialdin-Khaffaf, S. N. & Lal, G. S. & Sharif, I. & Syvret, R. G. 1-Alkyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane salts: a novel family of electrophilic fluorinating agents. J. Chem. Soc. Chem. Commun. 0, 595–596 (1992).
Differding, E. & Ofner H. N-Fluorobenzenesulfonimide: a practical reagent for electrophilic fluorinations. Synlett.187–18 9 (1991)..
Nyffeler, P. T., Durón, S. G., Burkart, M. D., Vincent, S. P. & Wong, C.-H. Selectfluor: mechanistic insight and applications. Angew. Chem. Int. Ed. 44, 192–212 (2005).
Guo, S., Cong, F., Guo, R., Wang, L. & Tang, P. Asymmetric silver-catalysed intermolecular bromotrifluoromethoxylation of alkenes with a new trifluoromethoxylation reagent. Nat. Chem. 9, 546–551 (2017).
Yamamoto, K. et al. Palladium-catalysed electrophilic aromatic C–H fluorination. Nature 554, 511–514 (2018).
Saadi, J. & Wennemers, H. Enantioselective aldol reactions with masked fluoroacetates. Nat. Chem. 8, 276–280 (2016).
Liang, T., Neumann, C. N. & Ritter, T. Introduction of fluorine and fluorine-containing functional groups. Angew. Chem. Int. Ed. 52, 8214–8264 (2013).
Peng, J. & Du, D.-M. Efficient enantioselective fluorination of β-keto esters/amides catalysed by diphenylamine-linked bis(thiazoline)–Cu(OTf)2 complexes. RSC Adv. 4, 2061–2067 (2013).
Li, F., Wu, Z. & Wang, J. Oxidative enantioselective α-fluorination of aliphatic aldehydes enabled by N-heterocyclic carbene catalysis. Angew. Chem. Int. Ed. 54, 656–659 (2015).
Beeson, T. D. & MacMillan, D. W. C. Enantioselective organocatalytic α-fluorination of aldehydes. J. Am. Chem. Soc. 127, 8826–8828 (2005).
Paull, D. H., Scerba, M. T., Alden-Danforth, E., Widger, L. R. & Lectka, T. Catalytic, asymmetric α-fluorination of acid chlorides: dual metal−ketene enolate activation. J. Am. Chem. Soc. 130, 17260–17261 (2008).
Wheeler, P., Vora, H. U. & Rovis, T. Asymmetric NHC-catalyzed synthesis of α-fluoroamides from readily accessible α-fluoroenals. Chem. Sci. 4, 1674–1679 (2013).
Dong, X., Yang, W., Hu, W. & Sun, J. N-Heterocyclic carbene catalyzed enantioselective α-fluorination of aliphatic aldehydes and α-chloro aldehydes: synthesis of α-fluoro esters, amides, and thioesters. Angew. Chem. Int. Ed. 54, 660–663 (2015).
Wu, J. Review of recent advances in nucleophilic C–F bond-forming reactions at sp 3 centers. Tetrahedron Lett. 55, 4289–4294 (2014).
Hollingworth, C. & Gouverneur, V. Transition metal catalysis and nucleophilic fluorination. Chem. Commun. 48, 2929–2942 (2012).
Neumann, C. N., Hooker, J. M. & Ritter, T. Concerted nucleophilic aromatic substitution with 19F− and 18F−. Nature 534, 369–373 (2016).
Liu, Z. et al. An organotrifluoroborate for broadly applicable one-step 18F-labeling. Angew. Chem. Int. Ed. 53, 11876–11880 (2014).
Campbell, M. G. et al. Bridging the gaps in 18F PET tracer development. Nat. Chem. 9, 1–3 (2017).
Jacobson, O., Kiesewetter, D. O. & Chen, X. Fluorine-18 Radiochemistry, Labeling Strategies and Synthetic Routes. Bioconjug. Chem. 26, 1–18 (2015).
Neumann, C. N. & Ritter, T. Late-stage fluorination: fancy novelty or useful tool? Angew. Chem. Int. Ed. 54, 3216–3221 (2015).
Falmagne, J.-B., Escudero, J., Taleb-Sahraoui, S. & Ghosez, L. Cyclobutanone and cyclobutenone derivatives by reaction of tertiary amides with alkenes or alkynes. Angew. Chem. Int. Ed. Engl. 20, 879–880 (1981).
Charette, A. B. & Grenon, M. Spectroscopic studies of the electrophilic activation of amides with triflic anhydride and pyridine. Can. J. Chem. 79, 1694–1703 (2001).
Movassaghi, M. & Hill, M. D. Synthesis of substituted pyridine derivatives via the ruthenium-catalyzed cycloisomerization of 3-azadienynes. J. Am. Chem. Soc. 128, 4592–4593 (2006).
Da Costa, R., Gillard, M., Falmagne, J. B. & Ghosez, L. α,β Dehydrogenation of carboxamides. J. Am. Chem. Soc. 101, 4381–4383 (1979).
Kaiser, D., de la Torre, A., Shaaban, S. & Maulide, N. Metal-free formal oxidative C−C coupling by in situ generation of an enolonium species. Angew. Chem. Int. Ed. 56, 5921–5925 (2017).
Kaiser, D., Teskey, C. J., Adler, P. & Maulide, N. Chemoselective intermolecular cross-enolate-type coupling of amides. J. Am. Chem. Soc. 139, 16040–16043 (2017).
Charette, A. B. & Chua, P. A new mild method for the cleavage of the amide bond: conversion of secondary and tertiary amides to esters. Synlett 1998, 163–165 (1998).
Gyoung, Y. S., Ko, S. H. & Yoon, N. M. A convenient procedure for the conversion of tertiary amides to the corresponding alcohols with lithium aluminum hydride. J. Korean Chem. Soc. 35, 296–298 (1991).
Liu, C., Achtenhagen, M. & Szostak, M. Chemoselective ketone synthesis by the addition of organometallics to N-acylazetidines. Org. Lett. 18, 2375–2378 (2016).
Otte, C. et al. Major depressive disorder. Nat. Rev. Dis. Primers 2, 16065 (2016).
Ionescu, D. F. & Papakostas, G. I. Experimental medication treatment approaches for depression. Transl. Psychiatry 7, e1068 (2017).
Alarcón, R. D. et al. Antidepressants: Past, Present, and Future (Springer, New York, NY, 2004).
Cipriani, A. et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet 391, 1357–1366 (2018).
Dahlmann, H. A. Spotlight. Chem. Res. Toxicol. 26, 1776–1777 (2013).
Fallahi-Sichani, M., Honarnejad, S., Heiser, L. M., Gray, J. W. & Sorger, P. K. Metrics other than potency reveal systematic variation in responses to cancer drugs. Nat. Chem. Biol. 9, 708–714 (2013).
Coleman, J. A., Green, E. M. & Gouaux, E. X-ray structures and mechanism of the human serotonin transporter. Nature 532, 334–339 (2016).
Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. Chembiochem 5, 570–589 (2004).
Brown, J. K. M. & Evans, N. Selection on responses of barley powdery mildew to morpholine and piperidine fungicides. Crop. Prot. 11, 449–457 (1992).
Volpe, D. A. et al. Uniform assessment and ranking of opioid Mu receptor binding constants for selected opioid drugs. Regul. Toxicol. Pharmacol. 59, 385–390 (2011).
Higashikawa, Y. & Suzuki, S. Studies on 1-(2-phenethyl)-4-(N-propionylanilino)piperidine (fentanyl) and its related compounds. VI. Structure–analgesic activity relationship for fentanyl, methyl-substituted fentanyls and other analogues. Forensic Toxicol. 26, 1–5 (2008).
Frank, R. G. & Pollack, H. A. Addressing the fentanyl threat to public health. N. Engl. J. Med. 376, 605–607 (2017).
Acknowledgements
A. Roller (University of Vienna) is thanked for X-ray crystallographic determination, E. Macoratti (University of Vienna) for preparative HPLC and G. Di Mauro for assistance with LigandScout. The authors thank the University of Vienna for continued support of their research programmes. Financial support by the Austrian Research Fund/FWF (grant F3506 to H.S.S.; grant M2274 to C.J.T.; grant P30226 to N.M.) and the Austrian Academy of Sciences (DOC-fellowship to D.K.) is acknowledged.
Author information
Authors and Affiliations
Contributions
P.A., C.J.T and D.K. performed and analysed the chemical experiments. P.A., C.J.T and N.M. co-wrote the manuscript. M.H and H.H.S. performed the biological testing. N.M. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary optimization data, experimental details and compound characterization data
Crystallographic data
CIF for compound (S,S)−9-L-DBTA; CCDC reference: 1866533
Rights and permissions
About this article
Cite this article
Adler, P., Teskey, C.J., Kaiser, D. et al. α-Fluorination of carbonyls with nucleophilic fluorine. Nat. Chem. 11, 329–334 (2019). https://doi.org/10.1038/s41557-019-0215-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-019-0215-z
This article is cited by
-
Design, synthesis, crystal structure, and in vitro antibacterial activities of sulfonamide derivatives bearing the 4-aminoquinazoline moiety
Molecular Diversity (2023)
-
Enzymatic synthesis of fluorinated compounds
Applied Microbiology and Biotechnology (2021)