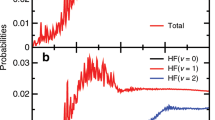
Abstract
Intersystem crossing plays an important role in photochemistry. It is understood to be efficient when heavy atoms are present due to strong spin–orbit coupling, or when strongly bound long-lived complexes are formed that increase the chance of finding the singlet–triplet intersection seam. Here we present evidence for a different intersystem crossing mechanism in the bimolecular reaction of O(3P) with alkylamines. In crossed-beam experiments, product velocity–flux maps are measured for aminoalkyl radicals produced from H abstraction from the methyl group, which also gives OH radicals as co-fragments. The low translational-energy release and isotropic angular distributions of the products indicate that such reactions undergo the formation of a complex before OH and aminoalkyl are produced. However, there is no well on the triplet potential energy surface that could support such a complex. Multi-reference ab initio calculations suggest, instead, that intersystem crossing occurs in the exit-channel region due to the long-range dipole–dipole interaction between the nascent radical product pair coupled with the vanishing singlet–triplet splitting at long range. Intersystem crossing then leads to a deep hydroxylamine well before OH elimination.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The authors confirm that all relevant data are included in the paper and/or its Supplementary Information, except raw image data, which are available on reasonable request from the authors.
References
Bolton, O., Lee, K., Kim, H. J., Lin, K. Y. & Kim, J. Activating efficient phosphorescence from purely organic materials by crystal design. Nat. Chem. 3, 205–210 (2011).
Goushi, K., Yoshida, K., Sato, K. & Adachi, C. Organic light-emitting diodes employing efficient reverse intersystem crossing for triplet-to-singlet state conversion. Nat. Photon. 6, 253–258 (2012).
Zhao, J. Z., Wu, W. H., Sun, J. F. & Guo, S. Triplet photosensitizers: from molecular design to applications. Chem. Soc. Rev. 42, 5323–5351 (2013).
Kamkaew, A. et al. BODIPY dyes in photodynamic therapy. Chem. Soc. Rev. 42, 77–88 (2013).
Koziar, J. C. & Cowan, D. O. Photochemical heavy-atom effects. Acc. Chem. Res. 11, 334–341 (1978).
Alagia, M. et al. Crossed beam studies of the O(3P,1D) + CH3I reactions: direct evidence of intersystem crossing. Faraday Discuss. 113, 133–150 (1999).
Casavecchia, P., Leonori, F. & Balucani, N. Reaction dynamics of oxygen atoms with unsaturated hydrocarbons from crossed molecular beam studies: primary products, branching ratios and role of intersystem crossing. Int. Rev. Phys. Chem. 34, 161–204 (2015).
Fu, B. N. et al. Intersystem crossing and dynamics in O(3P) + C2H4 multichannel reaction: experiment validates theory. Proc. Natl Acad. Sci. USA 109, 9733–9738 (2012).
Leonori, F. et al. Experimental and theoretical studies on the dynamics of the O(3P) + propene reaction: primary products, branching ratios and role of intersystem crossing. J. Phys. Chem. C 119, 14632–14652 (2015).
Leonori, F. et al. Crossed molecular beam dynamics studies of the O(3P) + allene reaction: primary products, branching ratios and dominant role of intersystem crossing. J. Phys. Chem. Lett. 3, 75–80 (2012).
Schmoltner, A. M., Chu, P. M., Brudzynski, R. J. & Lee, Y. T. Crossed molecular beam study of the reaction O(3P) + C2H4. J. Chem. Phys. 91, 6926–6936 (1989).
Schmoltner, A. M., Huang, S. Y., Brudzynski, R. J., Chu, P. M. & Lee, Y. T. Crossed molecular-beam study of the reaction O(3P) + allene. J. Chem. Phys. 99, 1644–1653 (1993).
Joalland, B. et al. Dynamics of chlorine atom reactions with hydrocarbons: insights from imaging the radical product in crossed beams. J. Phys. Chem. A 118, 9281–9295 (2014).
Li, W., Chambreau, S. D., Lahanker, S. A. & Suits, A. G. Megapixel ion imaging with standard video. Rev. Sci. Instrum. 76, 063106 (2005).
Montgomery, J. A., Frisch, M. J., Ochterski, J. W. & Petersson, G. A. A complete basis set model chemistry. VII. Use of the minimum population localization method. J. Chem. Phys. 112, 6532–6542 (2000).
Frisch, M. J. et al. Gaussian 09 Revision D.01 (Gaussian, 2013).
Joalland, B., Shi, Y., Kamasah, A., Suits, A. G. & Mebel, A. M. Roaming dynamics in radical addition-elimination reactions. Nat. Commun. 5, 4064 (2014).
Slagle, I. R., Dudich, J. F. & Gutman, D. Identification of reactive routes in the reactions of oxygen atoms with methylamine, dimethylamine, trimethylamine, ethylamine, diethylamine and triethylamine. J. Phys. Chem. 83, 3065–3070 (1979).
Atkinson, R. & Pitts, J. N. Kinetics of the reactions of O(3P) atoms with the amines CH3NH2, C2H5NH2, (CH3)2NH, and (CH3)3N over the temperature range 298–440 °K. J. Chem. Phys. 68, 911–915 (1978).
Balucani, N., Leonori, F., Casavecchia, P., Fu, B. N. & Bowman, J. M. Crossed molecular beams and quasiclassical trajectory surface hopping studies of the multichannel nonadiabatic O(3P) + ethylene reaction at high collision energy. J. Phys. Chem. A 119, 12498–12511 (2015).
Schmidt, M. W. et al. General atomic and molecular electronic structure system. J. Comput. Chem. 14, 1347–1363 (1993).
Gordon, M. S. & Schmidt, M. W. in Theory and Applications of Computational Chemistry: The First Forty Years (eds Dykstra, D. E., Frenking, K. S. K. & Scuseria, G. E.) 1167–1189 (Elsevier, Amsterdam, 2005).
Nakano, H. Quasidegenerate perturbation theory with multiconfigurational self‐consistent‐field reference functions. J. Chem. Phys. 99, 7983–7992 (1993).
Nakano, H. MCSCF reference quasidegenerate perturbation theory with Epstein–Nesbet partitioning. Chem. Phys. Lett. 207, 372–378 (1993).
El‐Sayed, M. A. Spin–orbit coupling and the radiationless processes in nitrogen heterocyclics. J. Chem. Phys. 38, 2834–2838 (1963).
Zobel, J. P., Nogueira, J. J. & González, L. Mechanism of ultrafast intersystem crossing in 2-nitronaphthalene. Chem. Eur. J. 24, 5379–5387 (2018).
Ahmed, M., Peterka, D. S. & Suits, A. G. Imaging H abstraction dynamics in crossed molecular beams: Cl + ROH reactions. Phys. Chem. Chem. Phys. 2, 861–868 (2000).
Huang, C., Li, W. & Suits, A. G. Rotationally resolved reactive scattering: imaging detailed Cl+C2H6 reaction dynamics. J. Chem. Phys. 125, 133107 (2006).
Abeysekera, C. et al. Note: a short-pulse high-intensity molecular beam valve based on a piezoelectric stack actuator. Rev. Sci. Instrum. 85, 116107 (2014).
Kawasaki, M. & Sato, H. Photodissociation of molecular beams of SO2 at 193 nm. Chem. Phys. Lett. 139, 585–588 (1987).
Felder, P., Effenhauser, C. S., Haas, B. M. & Huber, J. R. Photodissociation of sulfur dioxide at 193 nm. Chem. Phys. Lett. 148, 417–422 (1988).
Acknowledgements
The experimental research (A.G.S.) was supported by the Director, Office of Science, Office of Basic Energy Science, Division of Chemical Science, Geoscience and Bioscience of the US Department of Energy under contract no. DE-SC0017130 with additional support from the ARO under grant no. W911NF-17-1-0099. S.M. was supported by the NSF (CHE-1800171). The authors thank J. M. Bowman for comments on the manuscript and T. Sewell for helpful discussions.
Author information
Authors and Affiliations
Contributions
H.L. and A.K. performed the experiments and analysed the data. H.L. and S.M. performed the theoretical calculations and analysed the results. A.G.S. conceived the experiments and guided the interpretation. H.L., S.M. and A.G.S. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Results, Supplementary Figures 1 and 2, key stationary point geometries
Supplementary Movie
This video contains animations of one trajectory starting from the transition state of the direct H-abstraction process to OH + CH3NHCH2 products on the triplet surface, calculated using a Born–Oppenheimer molecular dynamics model at the B3LYP/6–31G(d) level of theory. Colour coding for atoms: carbon (grey), nitrogen (blue), oxygen (red) and hydrogen (white). A few snapshotsfor this trajectory are shown in Fig. 5.
Rights and permissions
About this article
Cite this article
Li, H., Kamasah, A., Matsika, S. et al. Intersystem crossing in the exit channel. Nature Chem 11, 123–128 (2019). https://doi.org/10.1038/s41557-018-0186-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0186-5
This article is cited by
-
Intersystem crossing in the entrance channel of the reaction of O(3P) with pyridine
Nature Chemistry (2022)
-
Radical pairs may play a role in microtubule reorganization
Scientific Reports (2022)
-
Unexpected intersystem crossing
Nature Chemistry (2019)