Abstract
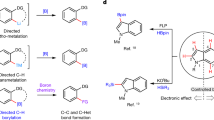
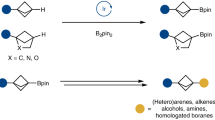
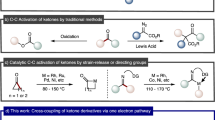
Transition-metal-catalysed cross-coupling reactions, particularly those mediated by palladium, are some of the most broadly used chemical transformations. The fundamental reaction steps of such cross-couplings typically include oxidative addition, transmetallation, carbopalladation of a π-bond and/or reductive elimination. Herein, we describe an unprecedented fundamental reaction step: a C–C σ-bond carbopalladation. Specifically, an aryl palladium(ii) complex interacts with a σ-bond of a strained bicyclo[1.1.0]butyl boronate complex to enable addition of the aryl palladium(ii) species and an organoboronic ester substituent across a C–C σ-bond. The overall process couples readily available aryl triflates and organoboronic esters across a cyclobutane unit with total diastereocontrol. The pharmaceutically relevant 1,1,3-trisubstituted cyclobutane products are decorated with an array of modular building blocks, including a boronic ester that can be readily derivatized.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information. Crystallographic data for compounds 5, 7, 15 and 35 are available free of charge from the Cambridge Crystallographic Date Centre (www.ccdc.cam.ac.uk) under reference numbers 1835072, 1847415, 1835073 and 1847416, respectively.
References
de Meijere, A., Bräse, S. & Oestreich, M. (eds) Metal-Catalyzed Cross-Coupling Reactions and More (Wiley, New York, 2013).
Cooper, T. W. J., Campbell, I. B. & Macdonald, S. J. F. Factors determining the selection of organic reactions by medicinal chemists and the use of these reactions in arrays (small focused libraries). Angew. Chem. Int. Ed. 49, 8082–8091 (2010).
Blakemore, D. C. et al. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 10, 383–394 (2018).
Miyaura, N. & Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 95, 2457–2483 (1995).
Zhang, L. et al. Catalytic conjunctive cross-coupling enabled by metal-induced metalate rearrangement. Science 351, 70–74 (2016).
Lovinger, G. J., Aparece, M. D. & Morken, J. P. Pd-catalyzed conjunctive cross-coupling between Grignard-derived boron ‘ate’ complexes and C(sp 2) halides or triflates: NaOTf as a Grignard activator and halide scavenger. J. Am. Chem. Soc. 139, 3153–3160 (2017).
Edelstein, E. K., Namirembe, S. & Morken, J. P. Enantioselective conjunctive cross-coupling of bis(alkenyl)borates: a general synthesis of chiral allylboron reagents. J. Am. Chem. Soc. 139, 5027–5030 (2017).
Chierchia, M., Law, C. & Morken, J. P. Ni-catalyzed enantioselective conjunctive cross-coupling of 9-BBN borates. Angew. Chem. Int. Ed. 56, 11870–11874 (2017).
Lovinger, G. J. & Morken, J. P. Ni-catalyzed enantioselective conjunctive coupling with C(sp 3) electrophiles: a radical-ionic mechanistic dichotomy. J. Am. Chem. Soc. 139, 17293–17296 (2017).
McDonald, R. I., Liu, G. & Stahl, S. S. Palladium(ii)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 111, 2981–3019 (2011).
Crabtree, R. H. Transition metal complexation of σ bonds. Angew. Chem. Int. Ed. Engl. 32, 789–805 (1993).
Gianatassio, R. et al. Strain-release amination. Science 351, 241–246 (2016).
Lopchuk, J. M. et al. Strain-release heteroatom functionalization: development, scope and stereospecificity. J. Am. Chem. Soc. 139, 3209–3226 (2017).
Murakami, M. & Chatani, N. (eds) Cleavage of Carbon–Carbon Single Bonds by Transition Metals (Wiley, New York, 2015).
Murakami, M. & Ishida, N. Potential of metal-catalyzed C–C single bond cleavage for organic synthesis. J. Am. Chem. Soc. 138, 13759–13769 (2016).
Souillart, L. & Cramer, N. Catalytic C–C bond activations via oxidative addition to transition metals. Chem. Rev. 115, 9410–9464 (2015).
Aoki, S., Fujimura, T., Nakamura, E. & Kuwajima, I. Palladium-catalyzed arylation of siloxycyclopropanes with aryl triflates. Carbon chain elongation via catalytic carbon–carbon bond cleavage. J. Am. Chem. Soc. 110, 3296–3298 (1988).
Chen, L. et al. Palladium-catalyzed ring-opening of 2-alkylidenecyclobutanols: stereoselective synthesis of γ,δ-unsaturated ketones by C–C bond cleavage. Adv. Synth. Catal. 360, 411–415 (2018).
Wiberg, K. B. et al. Bicyclo[1.1.0]butane. Tetrahedron 21, 2749–2769 (1965).
Khoury, P. R., Goddard, J. D. & Tam, W. Ring strain energies: substituted rings, norbornanes, norbornenes and norbornadienes. Tetrahedron 60, 8103–8112 (2004).
Walczak, M. A. A., Krainz, T. & Wipf, P. Ring-strain-enabled reaction discovery: new heterocycles from bicyclo[1.1.0]butanes. Acc. Chem. Res. 48, 1149–1158 (2015).
Walczak, M. A. A. & Wipf, P. Rhodium(i)-catalyzed cycloisomerizations of bicyclobutanes. J. Am. Chem. Soc. 130, 6924–6925 (2008).
Martín-Heras, V., Parra, A. & Tortosa, M. Cyclopropyl- and cyclobutylboronates and -silanes: a stereoselective approach. Synthesis 50, 470–484 (2018).
Poplata, S., Tröster, A., Zou, Y.-Q. & Bach, T. Recent advances in the synthesis of cyclobutanes by olefin [2 + 2] photocycloaddition reactions. Chem. Rev. 116, 9748–9815 (2016).
Gutekunst, W. R. & Baran, P. S. Applications of C–H functionalization logic to cyclobutane synthesis. J. Org. Chem. 79, 2430–2452 (2014).
Dembitsky, V. M. Naturally occurring bioactive cyclobutane-containing (CBC) alkaloids in fungi, fungal endophytes and plants. Phytomedicine 21, 1559–1581 (2014).
Blakemore, D. C. et al. Synthesis and in vivo evaluation of bicyclic gababutins. Bioorg. Med. Chem. Lett. 20, 461–464 (2010).
Slade, J. et al. A concise synthesis of a novel insulin-like growth factor I receptor (IGF-IR) inhibitor. Org. Process Res. Dev. 11, 825–835 (2007).
Namyslo, J. C. & Kaufmann, D. E. The application of cyclobutane derivatives in organic synthesis. Chem. Rev. 103, 1485–1537 (2003).
Seiser, T., Saget, T., Tran, D. N. & Cramer, N. Cyclobutanes in catalysis. Angew. Chem. Int. Ed. 50, 7740–7752 (2011).
Wrobleski, M. L. et al. Cyclobutane derivatives as potent NK1 selective antagonists. Bioorg. Med. Chem. Lett. 16, 3859–3863 (2006).
Stepan, A. P. et al. Application of the bicyclo[1.1.0]pentane motif as a nonclassical phenyl ring bioisostere in the design of a potent and orally active γ-secretase inhibitor. J. Med. Chem. 55, 3414–3424 (2012).
Nicolaou, K. C. et al. Synthesis and biopharmaceutical evaluation of imatinib analogues featuring unusual structural motifs. ChemMedChem 11, 31–37 (2016).
Blanco-Ania, D. et al. Rapid and scalable access into strained scaffolds through continuous flow photochemistry. Org. Process Res. Dev. 20, 409–413 (2016).
Casoni, G. et al. α-Sulfinyl benzoates as precursors to Li and Mg carbenoids for the stereoselective iterative homologation of boronic esters. J. Am. Chem. Soc. 139, 11877–11886 (2017).
Bottoni, A., Lombardo, M., Neri, A. & Trombini, C. Migratory aptitudes of simple alkyl groups in the anionotropic rearrangement of quaternary chloromethyl borate species: a combined experimental and theoretical investigation. J. Org. Chem. 68, 3397–3405 (2003).
Barreiro, E. J., Kümmerle, A. E. & Fraga, C. A. M. The methylation effect in medicinal chemistry. Chem. Rev. 111, 5215–5246 (2011).
Sandford, C. & Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 53, 5481–5494 (2017).
Bonet, A., Odachowski, M., Leonori, D., Essafi, S. & Aggarwal, V. K. Enantiospecific sp 2–sp 3 coupling of secondary and tertiary boronic esters. Nat. Chem. 6, 584–589 (2014).
Llaveria, J., Leonori, D. & Aggarwal, V. K. Stereospecific coupling of boronic esters with N-heteroaromatic compounds. J. Am. Chem. Soc. 137, 10958–10961 (2015).
Armstrong, R. J., Niwetmarin, W. & Aggarwal, V. K. Synthesis of functionalized alkenes by a transition-metal-free coupling. Org. Lett. 19, 2762–2765 (2017).
Wang, Y., Noble, A., Myers, E. L. & Aggarwal, V. K. Enantiospecific alkynylation of alkylboronic esters. Angew. Chem. Int. Ed. 55, 4270–4274 (2016).
Bagutski, V., Ros, A. & Aggarwal, V. K. Improved method for the conversion of pinacolboronic esters into trifluoroborate salts. Facile synthesis of chiral secondary and tertiary trifluoroborates. Tetrahedron 65, 9956–9960 (2009).
Molander, G. A. Organotrifluoroborates: another branch of the mighty oak. J. Org. Chem. 80, 7837–7848 (2015).
Nave, S., Sonawane, R. P., Elford, T. G. & Aggarwal, V. K. Protodeboronation of tertiary boronic esters: asymmetric synthesis of tertiary alkyl stereogenic centers. J. Am. Chem. Soc. 132, 17096–17098 (2010).
Fujimoto, H., Yabuki, T. & Fukui, K. A study of orbital interactions in the reactions of bicyclo[1.1.0]butane. J. Mol. Struct. 198, 267–275 (1989).
Newton, M. D. & Schulman, J. M. Theoretical studies of bicyclobutane. J. Am. Chem. Soc. 94, 767–773 (1972).
Leonori, D. & Aggarwal, V. K. Stereospecific couplings of secondary and tertiary boronic esters. Angew. Chem. Int. Ed. 54, 1082–1096 (2015).
Labadie, J. W. & Stille, J. K. Mechanisms of the palladium-catalyzed couplings of acid chlorides with organotin reagents. J. Am. Chem. Soc. 105, 6129–6137 (1983).
Sandrock, D. L., Jean-Gérard, L., Chen, C.-Y., Dreher, S. D. & Molander, G. A. Stereospecific cross-coupling of secondary alkyl b-trifluoratoamides. J. Am. Chem. Soc. 132, 17108–17110 (2010).
Hatanaka, Y. & Hiyama, T. Stereochemistry of the cross-coupling reaction of chiral alkylsilanes with aryl triflates: a novel approach to optically active compounds. J. Am. Chem. Soc. 112, 7793–7794 (1990).
Ohmura, T., Awano, T. & Suginome, M. Stereospecific Suzuki–Miyaura coupling of chiral α-(acylamino)benzylboronic esters with inversion of configuration. J. Am. Chem. Soc. 132, 13191–13193 (2010).
Acknowledgements
This work was supported by the EPSRC (EP/I038071/1), H2020 ERC (670668) and the Bayer Science and Education Foundation (Otto–Bayer Fellowship; T.B.). The authors thank E. L. Myers (NUI Galway) and A. Noble for helpful discussions, E. Denton for technical support and H. A. Sparkes for X-ray analysis.
Author information
Authors and Affiliations
Contributions
V.K.A. and A.F. conceived the project. A.F. designed and conducted the experiments and analysed the data. T.B. first synthesized compound 5. V.K.A. and A.F. prepared the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
Supplementary experimental details and compound characterization data
Crystallographic data
CIF for compound 5; CDCC reference 1835072
Crystallographic data
CIF for compound 7; CDCC reference 1847415
Crystallographic data
CIF for compound 15; CDCC reference 1835073
Crystallographic data
CIF for compound 35; CDCC reference 1847416
Rights and permissions
About this article
Cite this article
Fawcett, A., Biberger, T. & Aggarwal, V.K. Carbopalladation of C–C σ-bonds enabled by strained boronate complexes. Nature Chem 11, 117–122 (2019). https://doi.org/10.1038/s41557-018-0181-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0181-x
This article is cited by
-
Umpolung reactivity of strained C–C σ-bonds without transition-metal catalysis
Nature Communications (2024)
-
Decarboxylation of β-boryl NHPI esters enables radical 1,2-boron shift for the assembly of versatile organoborons
Nature Communications (2023)
-
Bicyclobutanes as unusual building blocks for complexity generation in organic synthesis
Communications Chemistry (2023)
-
Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer
Nature (2022)
-
How mono- and diphosphine ligands alter regioselectivity of the Rh-catalyzed annulative cleavage of bicyclo[1.1.0]butanes
Nature Communications (2022)