Abstract
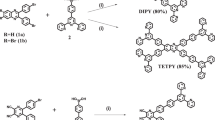
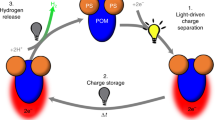
The oxygen in Earth’s atmosphere is there primarily because of water oxidation performed by photosynthetic organisms using solar light and one specialized protein complex, photosystem II (PSII). High-resolution imaging of the PSII ‘core’ complex shows the ideal co-localization of multi-chromophore light-harvesting antennas with the functional reaction centre. Man-made systems are still far from replicating the complexity of PSII, as the majority of PSII mimetics have been limited to photocatalytic dyads based on a 1:1 ratio of a light absorber, generally a Ru–polypyridine complex, with a water oxidation catalyst. Here we report the self-assembly of multi-perylene-bisimide chromophores (PBI) shaped to function by interaction with a polyoxometalate water-oxidation catalyst (Ru4POM). The resulting [PBI]5Ru4POM complex shows a robust amphiphilic structure and dynamic aggregation into large two-dimensional paracrystalline domains, a redshifted light-harvesting efficiency of >40% and favourable exciton accumulation, with a peak quantum efficiency using ‘green’ photons (λ > 500 nm). The modularity of the building blocks and the simplicity of the non-covalent chemistry offer opportunities for innovation in artificial photosynthesis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information files. All other relevant source data are available from the corresponding authors upon request.
Change history
13 March 2019
In the version of this Article originally published, in the graphical abstract the y-axis units of the plot read ‘mA cm–2’, but should have read ‘μA cm–2’. Additionally, an erroneous gap appeared in the red trace. These errors have now been corrected.
References
Ort, D. R. et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl Acad. Sci. USA 112, 8529–8536 (2015).
Blankenship, R. E. et al. Comparing photosynthetic and photovoltaic efficiencies and recognizing the potential for improvement. Science 332, 805–809 (2011).
Jia, J. et al. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 7, 13237 (2016).
Barber, J. Photosynthetic energy conversion: natural and artificial. Chem. Soc. Rev. 38, 185–196 (2009).
Emerson, R. & Arnold, W. The photochemical reaction in photosynthesis. J. Gen. Physiol. 16, 191–205 (1932).
Park, R. B. & Biggins, J. Quantasome: size and composition. Science 144, 1009–1011 (1964).
Kundu, S. & Patra, A. Nanoscale strategies for light harvesting. Chem. Rev. 117, 712–757 (2017).
Yamamoto, Y. et al. Efficient light harvesting via sequential two-step energy accumulation using a Ru–Re5 multinuclear complex incorporated into periodic mesoporous organosilica. Chem. Sci. 5, 639–648 (2014).
Croce, R. & Xu, P. A photo shoot of plant photosystem II. Nature 534, 42–43 (2016).
Yano, J. & Yachandra, V. Mn4Ca cluster in photosynthesis: where and how water is oxidized to dioxygen. Chem. Rev. 114, 4175–4205 (2014).
Kärkäs, M. D., Johnston, E. V., Verho, O. & Akermark, B. Artificial photosynthesis: from nanosecond electron transfer to catalytic water oxidation. Acc. Chem. Res. 47, 100–111 (2014).
Kärkäs, M. D., Verho, O., Johnston, E. V. & Åkermark, B. Artificial photosynthesis: molecular systems for catalytic water oxidation. Chem. Rev. 114, 11863–12001 (2014).
Berardi, S. et al. Molecular artificial photosynthesis. Chem. Soc. Rev. 43, 7501–7519 (2014).
Blakemore, J. D., Crabtree, R. H. & Brudvig, G. W. Molecular catalysts for water oxidation. Chem. Rev. 115, 12974–13005 (2015).
Young, K. J. et al. Light-driven water oxidation for solar fuels. Coord. Chem. Rev. 256, 2503–2520 (2012).
Sartorel, A., Bonchio, M., Campagna, S. & Scandola, F. Tetrametallic molecular catalysts for photochemical water oxidation. Chem. Soc. Rev. 42, 2262–2280 (2013).
Sartorel, A., Carraro, M., Toma, F. M., Prato, M. & Bonchio, M. Shaping the beating heart of artificial photosynthesis: oxygenic metal oxide nano-clusters. Energy Environ. Sci. 5, 5592 (2012).
Han, Q. & Ding, Y. Recent advances in the field of light-driven water oxidation catalyzed by transition-metal substituted polyoxometalates. Dalton Trans. 47, 8180–8188 (2018).
Gust, D., Moore, T. A. & Moore, A. L. Solar fuels via artificial photosynthesis. Acc. Chem. Res. 42, 1890–1898 (2009).
Gust, D. Supramolecular photochemistry applied to artificial photosynthesis and molecular logic devices. Faraday Discuss. 185, 9–35 (2015).
Fukuzumi, S., Ohkubo, K. & Suenobu, T. Long-lived charge separation and applications in artificial photosynthesis. Acc. Chem. Res. 47, 1455–1464 (2014).
Wasielewski, M. R. Self-assembly strategies for integrating light harvesting and charge separation in artificial photosynthetic systems. Acc. Chem. Res. 42, 1910–1921 (2009).
Cook, R. E. et al. Excimer formation and symmetry-breaking charge transfer in cofacial perylene dimers. J. Phys. Chem. A 121, 1607–1615 (2017).
Wu, Y. et al. Ultrafast photoinduced symmetry-breaking charge separation and electron sharing in perylenediimide molecular triangles. J. Am. Chem. Soc. 137, 13236–13239 (2015).
Würthner, F. et al. Perylene bisimide dye assemblies as archetype functional supramolecular materials. Chem. Rev. 116, 962–1052 (2015).
Chen, S., Slattum, P., Wang, C. & Zang, L. Self-assembly of perylene imide molecules into 1D nanostructures: methods, morphologies, and applications. Chem. Rev. 115, 11967–11998 (2015).
Weingarten, A. S. et al. Self-assembling hydrogel scaffolds for photocatalytic hydrogen production. Nat. Chem. 6, 964–970 (2014).
Guan, Y., Zakrevskyy, Y., Stumpe, J., Antonietti, M. & Faul, C. F. J. Perylenediimide-surfactant complexes: thermotropic liquid-crystalline materials via ionic self-assembly. Chem. Commun. 2003, 894–895 (2003).
Guan, Y., Yu, S.-H., Antonietti, M., Böttcher, C. & Faul, C. F. J. Synthesis of supramolecular polymers by ionic self-assembly of oppositely charged dyes. Chem. Eur. J. 11, 1305–1311 (2005).
Supur, M. & Fukuzumi, S. Photodriven electron transport within the columnar perylenediimide nanostructures self-assembled with sulfonated porphyrins in water. J. Phys. Chem. C 116, 23274–23282 (2012).
Supur, M. & Fukuzumi, S. Tuning the photodriven electron transport within the columnar perylenediimide stacks by changing the π-extent of the electron donors. Phys. Chem. Chem. Phys. 15, 2539 (2013).
Sartorel, A. et al. Water oxidation at a tetraruthenate core stabilized by polyoxometalate ligands: experimental and computational evidence to trace the competent intermediates. J. Am. Chem. Soc. 131, 16051–16053 (2009).
Toma, F. M. et al. Efficient water oxidation at carbon nanotube–polyoxometalate electrocatalytic interfaces. Nat. Chem. 2, 826–831 (2010).
Quintana, M. et al. Knitting the catalytic pattern of artificial photosynthesis to a hybrid graphene nanotexture. ACS Nano 7, 811–817 (2013).
Geletii, Y. V. et al. Structural, physicochemical, and reactivity properties of an all-inorganic, highly active tetraruthenium homogeneous catalyst for water oxidation. J. Am. Chem. Soc. 131, 17360–17370 (2009).
Liu, Y. et al. Voltammetric determination of the reversible potentials for [{Ru4O4(OH)2(H2O)4}(γ-SiW10O36)2]10− over the pH range of 2−12: electrolyte dependence and implications for water oxidation catalysis. Inorg. Chem. 4, 11986–11996 (2013).
Guo, S. X. et al. Graphene-supported [{Ru4O4(OH)2(H2O)4}(γ-SiW10O36)2]10− for highly efficient electrocatalytic water oxidation. Energy Environ. Sci. 6, 2654–2663 (2013).
Orlandi, M. et al. Ruthenium polyoxometalate water splitting catalyst: very fast hole scavenging from photogenerated oxidants. Chem. Commun. 46, 3152–3154 (2010).
Puntoriero, F. et al. Photo-induced water oxidation with tetra-nuclear ruthenium sensitizer and catalyst: a unique 4 × 4 ruthenium interplay triggering high efficiency with low-energy visible light. Chem. Commun. 46, 4725–4727 (2010).
Natali, M. et al. Working the other way around: photocatalytic water oxidation triggered by reductive quenching of the photoexcited chromophore. J. Phys. Chem. C 119, 2371–2379 (2015).
Vagnini, M. T. et al. Ultrafast photodriven intramolecular electron transfer from an iridium-based water-oxidation catalyst to perylene diimide derivatives. Proc. Natl Acad. Sci. USA 109, 15651–15656 (2012).
Piccinin, S. et al. Water oxidation surface mechanisms replicated by a totally inorganic tetraruthenium-oxo molecular complex. Proc. Natl Acad. Sci. USA 110, 4917–4922 (2013).
Scheuring, S. & Sturgis, J. N. Chromatic adaptation of photosynthetic membranes. Science 309, 484–487 (2005).
Nisar, A. & Wang, X. Surfactant-encapsulated polyoxometalate building blocks: controlled assembly and their catalytic properties. Dalton Trans. 41, 9832 (2012).
Seddon, J. M. Structure of the inverted hexagonal (HII) phase, and non-lamellar phase transitions of lipids. Biochim. Biophys. Acta 1031, 1–69 (1990).
Turner, D. C. & Gruner, S. M. X-ray diffraction reconstruction of the inverted hexagonal (HII) phase in lipid–water systems. Biochemistry 31, 1340–1355 (1992).
Parent, A. R., Crabtree, R. H. & Brudvig, G. W. Comparison of primary oxidants for water-oxidation catalysis. Chem. Soc. Rev. 42, 2247–2252 (2013).
Lai, Y.-H., Kato, M., Mersch, D. & Reisner, E. Comparison of photoelectrochemical water oxidation activity of a synthetic photocatalyst system with photosystem II. Faraday Discuss. 176, 199–211 (2014).
Swierk, J. R. & Mallouk, T. E. Design and development of photoanodes for water-splitting dye-sensitized photoelectrochemical cells. Chem. Soc. Rev. 42, 2357–2387 (2013).
Kim, J. Y. et al. Single-crystalline, wormlike hematite photoanodes for efficient solar water splitting. Sci. Rep. 3, 2681 (2013).
Kim, T. W. & Choi, K.-S. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science 343, 990–994 (2014).
Hegner, F. et al. Cobalt hexacyanoferrate on BiVO4 photoanodes for robust water splitting. ACS Appl. Mater. Interfaces 9, 37671–37681 (2017).
Scott, M. J., Nelson, J. J., Caramori, S., Bignozzi, C. A. & Elliott, C. M. cis-dichloro-bis(4,4′-dicarboxy-2,2-bipyridine)osmium(ii)-modified optically transparent electrodes: application as cathodes in stacked dye-sensitized solar cells. Inorg. Chem. 46, 10071–10078 (2007).
Hill, J. C. & Choi, K. S. Effect of electrolytes on the selectivity and stability of n-type WO3 photoelectrodes for use in solar water oxidation. J. Phys. Chem. C 116, 7612–7620 (2012).
Ronconi, F. et al. Modification of nanocrystalline WO3 with a dicationic perylene bisimide: applications to molecular level solar water splitting. J. Am. Chem. Soc. 137, 4630–4633 (2015).
Fielden, J. et al. Water splitting with polyoxometalate-treated photoanodes: enhancing performance through sensitizer design. Chem. Sci. 6, 5531–5543 (2015).
Natali, M. et al. Photo-assisted water oxidation by high-nuclearity cobalt-oxo cores: tracing the catalyst fate during oxygen evolution turnover. Green Chem. 19, 2416–2426 (2017).
Acknowledgements
This work was supported by the Italian Ministero dell’Istruzione, Università e Ricerca (FIRB RBAP11C58Y, PRIN-2010N3T9M4), the Universities of Padova and Trieste, INSTM, and the Seventh Framework Programme (FP7/2007–2013) under grant agreement no. 310651 (SACS project). Beamtime obtained at the facilities ELETTRA- Sincrotrone Trieste S.C.p.A. and CERIC-ERIC consortium is acknowledged.
Author information
Authors and Affiliations
Contributions
Z.S., E.P. and F.R. carried out the synthesis and characterization experiments. M.Burian and H.A. conducted the WAXS and SAXS structural investigations. N.M. isolated and characterized the hexagonal crystalline aggregates. N.M. and N.D. conducted the X-ray diffraction analysis. K.D. and D.M.G. designed and analysed the fsTA studies. G.A.V. and G.L. optimized the oxygenic activity and photocurrent performance. S.B., S.C. and C.A.B. conducted the photoelectrochemical cell experiments. A.S. analysed the solution and photoelectrochemical cell results. M.Bonchio and M.P. designed the experiments and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials and Methods, Supplementary Figs 1–18, Supplementary Tables 1 and 2
Rights and permissions
About this article
Cite this article
Bonchio, M., Syrgiannis, Z., Burian, M. et al. Hierarchical organization of perylene bisimides and polyoxometalates for photo-assisted water oxidation. Nature Chem 11, 146–153 (2019). https://doi.org/10.1038/s41557-018-0172-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0172-y
This article is cited by
-
Patterned immobilization of polyoxometalate-loaded mesoporous silica particles via amine-ene Michael additions on alkene functionalized surfaces
Scientific Reports (2024)
-
Catalyst self-assembly accelerates bimetallic light-driven electrocatalytic H2 evolution in water
Nature Chemistry (2024)
-
Artificial spherical chromatophore nanomicelles for selective CO2 reduction in water
Nature Catalysis (2023)
-
Coupled reaction equilibria enable the light-driven formation of metal-functionalized molecular vanadium oxides
Nature Communications (2023)
-
Supramolecular framework membrane for precise sieving of small molecules, nanoparticles and proteins
Nature Communications (2023)