Abstract
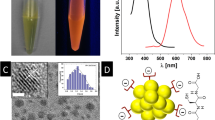
Although N-heterocyclic carbenes (NHCs) have demonstrated outstanding potential for use as surface anchors, synthetic challenges have limited their application to either large planar substrates or very small spherical nanoparticles. The development of a strategy to graft NHCs onto non-spherical nanomaterials, such as gold nanorods, would greatly expand their utility as surface ligands. Here, we use a bidentate thiolate–NHC–gold(i) complex that is easily grafted onto commercial cetyl trimethylammonium bromide-stabilized gold nanorods through ligand exchange. On mild reduction of the resulting surface-tethered NHC–gold(i) complexes, the gold atom attached to the NHC complex is added to the surface as an adatom, thereby precluding the need for reorganization of the underlying surface lattice upon NHC binding. The resulting thiolate–NHC-stabilized gold nanorods are stable towards excess glutathione for up to six days, and under conditions with large variations in pH, high and low temperatures, high salt concentrations, or in biological media and cell culture. We also demonstrate the utility of these nanorods for in vitro photothermal therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information, and/or from the corresponding author upon reasonable request.
References
Zhukhovitskiy, A. V., MacLeod, M. J. & Johnson, J. A. Carbene ligands in surface chemistry: from stabilization of discrete elemental allotropes to modification of nanoscale and bulk substrates. Chem. Rev. 115, 11503–11532 (2015).
Hopkinson, M. N., Richter, C., Schedler, M. & Glorius, F. An overview of N-heterocyclic carbenes. Nature 510, 485–496 (2014).
Ranganath, K. V. S., Kloesges, J., Schäfer, A. H. & Glorius, F. Asymmetric nanocatalysis: N-heterocyclic carbenes as chiral modifiers of Fe3O4/Pd nanoparticles. Angew. Chem. Int. Ed. 49, 7786–7789 (2010).
Ranganath, K. V. S., Schäfer, A. H. & Glorius, F. Comparison of superparamagnetic Fe3O4-supported N-heterocyclic carbene-based catalysts for enantioselective allylation. ChemCatChem 3, 1889–1891 (2011).
Serpell, C. J., Cookson, J., Thompson, A. L., Brown, C. M. & Beer, P. D. Haloaurate and halopalladate imidazolium salts: structures, properties, and use as precursors for catalytic metal nanoparticles. Dalton Trans. 42, 1385–1393 (2013).
Richter, C., Schaepe, K., Glorius, F. & Ravoo, B. J. Tailor-made N-heterocyclic carbenes for nanoparticle stabilization. Chem. Commun. 50, 3204–3207 (2014).
Ferry, A. et al. Negatively charged N-heterocyclic carbene-stabilized Pd and Au nanoparticles and efficient catalysis in water. ACS Catal. 5, 5414–5420 (2015).
Rühling, A. et al. Modular bidentate hybrid NHC–thioether ligands for the stabilization of palladium nanoparticles in various solvents. Angew. Chem. Int. Ed. 55, 5856–5860 (2016).
Soulé, J.-F., Miyamura, H. & Kobayashi, S. Copolymer-incarcerated nickel nanoparticles with N-heterocyclic carbene precursors as active cross-linking agents for Corriu–Kumada–Tamao reaction. J. Am. Chem. Soc. 135, 10602–10605 (2013).
de los Bernardos, M. D., Pérez-Rodríguez, S., Gual, A., Claver, C. & Godard, C. Facile synthesis of NHC-stabilized Ni nanoparticles and their catalytic application in the Z-selective hydrogenation of alkynes. Chem. Commun. 53, 7894–7897 (2017).
Lara, P., Suárez, A., Collière, V., Philippot, K. & Chaudret, B. Platinum N-heterocyclic carbene nanoparticles as new and effective catalysts for the selective hydrogenation of nitroaromatics. ChemCatChem 6, 87–90 (2014).
Lara, P. et al. Ruthenium nanoparticles stabilized by N-heterocyclic carbenes: ligand location and influence on reactivity. Angew. Chem. Int. Ed. 50, 12080–12084 (2011).
Gonzalez-Galvez, D. et al. NHC-stabilized ruthenium nanoparticles as new catalysts for the hydrogenation of aromatics. Catal. Sci. Tech. 3, 99–105 (2013).
Lara, P. et al. NHC-stabilized Ru nanoparticles: synthesis and surface studies. Nano-Structures Nano-Objects 6, 39–45 (2016).
Martínez-Prieto, L. M. et al. Long-chain NHC-stabilized RuNPs as versatile catalysts for one-pot oxidation/hydrogenation reactions. Chem. Commun. 52, 4768–4771 (2016).
Ernst, J. B., Muratsugu, S., Wang, F., Tada, M. & Glorius, F. Tunable heterogeneous catalysis: N-heterocyclic carbenes as ligands for supported heterogeneous Ru/K-Al2O3 catalysts to tune reactivity and selectivity. J. Am. Chem. Soc. 138, 10718–10721 (2016).
Song, S. G. et al. N-Heterocyclic carbene-based conducting polymer–gold nanoparticle hybrids and their catalytic application. Macromolecules 47, 6566–6571 (2014).
Crespo, J. et al. Ultrasmall NHC-coated gold nanoparticles obtained through solvent free thermolysis of organometallic Au(i) complexes. Dalton Trans. 43, 15713–15718 (2014).
Cao, Z. et al. A molecular surface functionalization approach to tuning nanoparticle electrocatalysts for carbon dioxide reduction. J. Am. Chem. Soc. 138, 8120–8125 (2016).
Ye, R. et al. Supported Au nanoparticles with N-heterocyclic carbene ligands as active and stable heterogeneous catalysts for lactonization. J. Am. Chem. Soc. 140, 4144–4149 (2018).
MacLeod, M. J. & Johnson, J. A. PEGylated N-heterocyclic carbene anchors designed to stabilize gold nanoparticles in biologically relevant media. J. Am. Chem. Soc. 137, 7974–7977 (2015).
Salorinne, K. et al. Water-soluble N-heterocyclic carbene-protected gold nanoparticles: size-controlled synthesis, stability, and optical properties. Angew. Chem. Int. Ed. 56, 6198–6202 (2017).
Zhukhovitskiy, A. V., Mavros, M. G., Van Voorhis, T. & Johnson, J. A. Addressable carbene anchors for gold surfaces. J. Am. Chem. Soc. 135, 7418–7421 (2013).
Crudden, C. M. et al. Ultra stable self-assembled monolayers of N-heterocyclic carbenes on gold. Nat. Chem. 6, 409–414 (2014).
Crudden, C. M. et al. Simple direct formation of self-assembled N-heterocyclic carbene monolayers on gold and their application in biosensing. Nat. Commun. 7, 12654 (2016).
Zhukhovitskiy, A. V. et al. Reactions of persistent carbenes with hydrogen-terminated silicon surfaces. J. Am. Chem. Soc. 138, 8639–8652 (2016).
Mackey, M. A., Ali, M. R. K., Austin, L. A., Near, R. D. & El-Sayed, M. A. The most effective gold nanorod size for plasmonic photothermal therapy: theory and in vitro experiments. J. Phys. Chem. B 118, 1319–1326 (2014).
Grzelczak, M., Pérez-Juste, J., Mulvaney, P. & Liz-Marzán, L. M. Shape control in gold nanoparticle synthesis. Chem. Soc. Rev. 37, 1783–1791 (2008).
Hurst, E. C., Wilson, K., Fairlamb, I. J. S. & Chechik, V. N-Heterocyclic carbene coated metal nanoparticles. New J. Chem. 33, 1837–1840 (2009).
Möller, N. et al. Stabilization of high oxidation state upconversion nanoparticles by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 56, 4356–4360 (2017).
Vignolle, J. & Tilley, T. D. N-Heterocyclic carbene-stabilized gold nanoparticles and their assembly into 3D superlattices. Chem. Commun. 2009, 7230–7232 (2009).
Ling, X., Roland, S. & Pileni, M. P. Supracrystals of N-heterocyclic carbene-coated Au nanocrystals. Chem. Mater. 27, 414–423 (2015).
Bridonneau, N. et al. N-Heterocyclic carbene-stabilized gold nanoparticles with tunable sizes. Dalton Trans. 47, 6850–6859 (2018).
Torrelles, X. et al. Solving the long-standing controversy of long-chain alkanethiols surface structure on Au(111). J. Phys. Chem. C 122, 3893–3902 (2018).
Rodríguez-Castillo, M. et al. Reactivity of gold nanoparticles towards N-heterocyclic carbenes. Dalton Trans. 43, 5978–5982 (2014).
Wang, G. Q. et al. Ballbot-type motion of N-heterocyclic carbenes on gold surfaces. Nat. Chem. 9, 152–156 (2017).
Jiang, L. et al. N-Heterocyclic carbenes on close-packed coinage metal surfaces: bis-carbene metal adatom bonding scheme of monolayer films on Au, Ag and Cu. Chem. Sci. 8, 8301–8308 (2017).
Collado, A., Gomez-Suarez, A., Martin, A. R., Slawin, A. M. Z. & Nolan, S. P. Straightforward synthesis of [Au(NHC)X] (NHC = N-heterocyclic carbene, X = Cl, Br, I) complexes. Chem. Commun. 49, 5541–5543 (2013).
Fèvre, M. et al. Imidazolium hydrogen carbonates versus imidazolium carboxylates as organic precatalysts for N-heterocyclic carbene catalyzed reactions. J. Org. Chem. 77, 10135–10144 (2012).
Li, Z., Jin, R. C., Mirkin, C. A. & Letsinger, R. L. Multiple thiol-anchor capped DNA-gold nanoparticle conjugates. Nucleic Acids Res. 30, 1558–1562 (2002).
Oh, E. et al. Colloidal stability of gold nanoparticles coated with multithiol-poly(ethylene glycol) ligands: importance of structural constraints of the sulfur anchoring groups. J. Phys. Chem. C 117, 18947–18956 (2013).
Oh, E., Susumu, K., Goswami, R. & Mattoussi, H. One-phase synthesis of water-soluble gold nanoparticles with control over size and surface functionalities. Langmuir 26, 7604–7613 (2010).
Man, R. W. Y. et al. Ultrastable gold nanoparticles modified by bidentate N-heterocyclic carbene ligands. J. Am. Chem. Soc. 140, 1576–1579 (2018).
Cao, Z. et al. Chelating N-heterocyclic carbene ligands enable tuning of electrocatalytic CO2 reduction to formate and carbon monoxide: surface organometallic chemistry. Angew. Chem., Int. Ed. 57, 4981–4985 (2018).
Pillar, V. N. R. Photo-removable protecting groups in organic-synthesis. Synthesis 1980, 1–26 1980).
Dreaden, E. C., Alkilany, A. M., Huang, X., Murphy, C. J. & El-Sayed, M. A. The golden age: gold nanoparticles for biomedicine. Chem. Soc. Rev. 41, 2740–2779 (2012).
Otsuka, H., Nagasaki, Y. & Kataoka, K. PEGylated nanoparticles for biological and pharmaceutical applications. Adv. Drug Deliv. Rev. 64, 246–255 (2012).
Locatelli, E., Monaco, I. & Franchini, M. C. Surface modifications of gold nanorods for applications in nanomedicine. RSC Adv. 5, 21681–21699 (2015).
Liu, H.-X., He, X. & Zhao, L. Metallamacrocycle-modified gold nanoparticles: a new pathway for surface functionalization. Chem. Commun. 50, 971–974 (2014).
Schulz, F. et al. Effective PEGylation of gold nanorods. Nanoscale 8, 7296–7308 (2016).
Khlebtsov, N. & Dykman, L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 40, 1647–1671 (2011).
Tang, Q. & Jiang, D. E. Comprehensive view of the ligand gold interface from first principles. Chem. Mater. 29, 6908–6915 (2017).
Schaefer, H. E. Investigation of thermal-equilibrium vacancies in metals by positron-annihilation. Phys. Status Solidi A 102, 47–65 (1987).
Qi, W. H. & Wang, M. P. Vacancy formation energy of small particles. J. Mater. Sci. 39, 2529–2530 (2004).
Huang, X. H., El-Sayed, I. H., Qian, W. & El-Sayed, M. A. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J. Am. Chem. Soc. 128, 2115–2120 (2006).
Huang, X. H. & El-Sayed, M. A. Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 1, 13–28 (2010).
Yang, X., Yang, M. X., Pang, B., Vara, M. & Xia, Y. N. Gold nanomaterials at work in biomedicine. Chem. Rev. 115, 10410–10488 (2015).
Doud, E. A. et al. In situ formation of N-heterocyclic carbene-bound single-molecule junctions. J. Am. Chem. Soc. 140, 8944–8949 (2018).
Acknowledgements
The authors thank the National Science Foundation (CHE-1351646) for support of this work.
Author information
Authors and Affiliations
Contributions
M.J.M. and J.A.J. conceived the idea. M.J.M. conducted all synthesis and characterization studies. A.J.G. conducted laser irradiation experiments. H.Y. and T.V.V. conducted DFT calculations. H.V.-T.N. conducted cell culture assays. M.J.M. and J.A.J. wrote the manuscript. All authors read and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Detailed methods and materials, experimental protocols, characterization data (such as spectral data), Supplementary Figures 1–45 and Supplementary Table 1
Rights and permissions
About this article
Cite this article
MacLeod, M.J., Goodman, A.J., Ye, HZ. et al. Robust gold nanorods stabilized by bidentate N-heterocyclic-carbene–thiolate ligands. Nature Chem 11, 57–63 (2019). https://doi.org/10.1038/s41557-018-0159-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0159-8
This article is cited by
-
N-Heterocyclic carbene-based C-centered Au(I)-Ag(I) clusters with intense phosphorescence and organelle-selective translocation in cells
Nature Communications (2022)
-
Recent advances in the chemistry and applications of N-heterocyclic carbenes
Nature Reviews Chemistry (2021)
-
N-Heterocyclic carbenes as tunable ligands for catalytic metal surfaces
Nature Catalysis (2021)
-
Effect of TAT-DOX-PEG irradiated gold nanoparticles conjugates on human osteosarcoma cells
Scientific Reports (2020)
-
Electrochemical deposition of N-heterocyclic carbene monolayers on metal surfaces
Nature Communications (2020)