Abstract
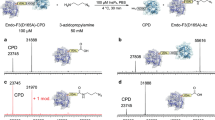
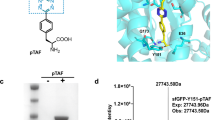
Conjugates between proteins and small molecules enable access to a vast chemical space that is not achievable with either type of molecule alone; however, the paucity of specific reactions capable of functionalizing proteins and natural products presents a formidable challenge for preparing conjugates. Here we report a strategy for conjugating electron-rich (hetero)arenes to polypeptides and proteins. Our bioconjugation technique exploits the electrophilic reactivity of an oxidized selenocysteine residue in polypeptides and proteins, and the electron-rich character of certain small molecules to provide bioconjugates in excellent yields under mild conditions. This conjugation chemistry enabled the synthesis of peptide–vancomycin conjugates without the prefunctionalization of vancomycin. These conjugates have an enhanced in vitro potency for resistant Gram-positive and Gram-negative pathogens. Additionally, we show that a 6 kDa affibody protein and a 150 kDa immunoglobulin-G antibody could be modified without diminishing bioactivity.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data generated or analysed during this study are included in this published article (and in the Supplementary Information). Further details are available from the corresponding authors upon request.
References
Drake, P. M. & Rabuka, D. An emerging playbook for antibody–drug conjugates: lessons from the laboratory and clinic suggest a strategy for improving efficacy and safety. Curr. Opin. Chem. Biol. 28, 174–180 (2015).
Ueda, T. Next-generation optimized biotherapeutics—a review and preclinical study. Biochim. Biophys. Acta 1844, 2053–2057 (2014).
Hamann, P. R. et al. Gemtuzumab ozogamicin, a potent and selective anti-CD33 antibody–calicheamicin conjugate for treatment of acute myeloid leukemia. Bioconjug. Chem. 13, 47–58 (2002).
Wang, L., Amphlett, G., Blättler, W. A., Lambert, J. M. & Zhang, W. Structural characterization of the maytansinoid–monoclonal antibody immunoconjugate, huN901–DM1, by mass spectrometry. Protein Sci. 14, 2436–2446 (2005).
Lewis Phillips, G. D. et al. Targeting HER2-positive breast cancer with trastuzumab–DM1, an antibody–cytotoxic drug conjugate. Cancer Res. 68, 9280–9290 (2008).
Junutula, J. R. et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat. Biotechnol. 26, 925–932 (2008).
Boswell, C. A. et al. Impact of drug conjugation on pharmacokinetics and tissue distribution of anti-STEAP1 antibody–drug conjugates in rats. Bioconjug. Chem. 22, 1994–2004 (2011).
Pillow, T. H. et al. Site-specific trastuzumab maytansinoid antibody–drug conjugates with improved therapeutic activity through linker and antibody engineering. J. Med. Chem. 57, 7890–7899 (2014).
Akkapeddi, P. et al. Construction of homogeneous antibody–drug conjugates using site-selective protein chemistry. Chem. Sci. 7, 2954–2963 (2016).
Zou, L.-H., Reball, J., Mottweiler, J. & Bolm, C. Transition metal-free direct C–H bond thiolation of 1,3,4-oxadiazoles and related heteroarenes. Chem. Commun. 48, 11307–11309 (2012).
Hostier, T., Ferey, V., Ricci, G., Gomez Pardo, D. & Cossy, J. Synthesis of aryl sulfides: metal-free C–H sulfenylation of electron-rich arenes. Org. Lett. 17, 3898–3901 (2015).
Ranjit, S. et al. Copper-mediated C–H activation/C–S cross-coupling of heterocycles with thiols. J. Org. Chem. 76, 8999–9007 (2011).
Fang, X.-L., Tang, R.-Y., Zhong, P. & Li, J.-H. Iron-catalyzed sulfenylation of indoles with disulfides promoted by a catalytic amount of iodine. Synthesis 2009, 4183–4189 (2009).
Cohen, D. T., Zhang, C., Pentelute, B. L. & Buchwald, S. L. An umpolung approach for the chemoselective arylation of selenocysteine in unprotected peptides. J. Am. Chem. Soc. 137, 9784–9787 (2015).
Hall, D. G. in Boronic Acids: Preparation and Applications in Organic Synthesis and Medicine (ed. Hall, D. G.) Ch. 1 (Wiley, Hoboken, 2006).
Small, P. M. & Chambers, H. F. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34, 1227–1231 (1990).
Cetinkaya, Y., Falk, P. & Mayhall, C. G. Vancomycin-resistant enterococci. Clin. Microbiol. Rev. 13, 686–707 (2000).
Navon-Venezia, S., Feder, R., Gaidukov, L., Carmeli, Y. & Mor, A. Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob. Agents Chemother. 46, 689–694 (2002).
Yount, N. Y. et al. Context mediates antimicrobial efficacy of kinocidin congener peptide RP-1. PLoS One 6, e26727 (2011).
Dijkshoorn, L., Nemec, A. & Seifert, H. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 5, 939–951 (2007).
O’Shea, M. K. Acinetobacter in modern warfare. Int. J. Antimicrob. Agents 39, 363–375 (2012).
Maragakis, L. L. & Perl, T. M. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 46, 1254–1263 (2008).
Andreadou, I., Menge, W. M. P. B., Commandeur, J. N. M., Worthington, E. A. & Vermeulen, N. P. E. Synthesis of novel Se-substituted selenocysteine derivatives as potential kidney selective prodrugs of biologically active selenol compounds: evaluation of kinetics of β-elimination reactions in rat renal cytosol. J. Med. Chem. 39, 2040–2046 (1996).
Andreadou, I., van de Water, B., Commandeur, J. N., Nagelkerke, F. J. & Vermeulen, N. P. Comparative cytotoxicity of 14 novel selenocysteine Se-conjugates in rat renal proximal tubular cells. Toxicol. Appl. Pharmacol. 141, 278–287 (1996).
Spallholz, J. E. On the nature of selenium toxicity and carcinostatic activity. Free Radic. Biol. Med. 17, 45–64 (1994).
Löfblom, J. et al. Affibody molecules: engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 584, 2670–2680 (2010).
Orlova, A. et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 66, 4339–4348 (2006).
Popp, M. W., Antos, J. M., Grotenbreg, G. M., Spooner, E. & Ploegh, H. L. Sortagging: a versatile method for protein labeling. Nat. Chem. Biol. 3, 707–708 (2007).
Russo, M. et al. Understanding genistein in cancer: the ‘good’ and the ‘bad’ effects: a review. Food Chem. 196, 589–600 (2016).
Thyer, R., Robotham, S. A., Brodbelt, J. S. & Ellington, A. D. Evolving tRNASec for efficient canonical incorporation of selenocysteine. J. Am. Chem. Soc. 137, 46–49 (2015).
Hofer, T., Thomas, J. D., Burke, T. R. & Rader, C. An engineered selenocysteine defines a unique class of antibody derivatives. Proc. Natl Acad. Sci. USA 105, 12451–12456 (2008).
Beasley, R., Pearce, N., Crane, J. & Burgess, C. Withdrawal of fenoterol and the end of the New Zealand asthma mortality epidemic. Int. Arch. Allergy Immunol. 107, 325–327 (1995).
Miyamoto, Y., Sakamoto, Y., Yoshida, N. & Baba, H. Efficacy of S-1 in colorectal cancer. Expert. Opin. Pharmacother. 15, 1761–1770 (2014).
Wiśniewski, K. & Car, H. (S)-3,5-DHPG: a review. CNS Drug. Rev. 8, 101–116 (2002).
Chan, G. M., Sharma, R., Price, D., Hoffman, R. S. & Nelson, L. S. Phentolamine therapy for cocaine-associated acute coronary syndrome (CAACS). J. Med. Toxicol. 2, 108–111 (2006).
Wong, G. W. K., . & Boyda, H. N. & Wright, J. M. Blood pressure lowering efficacy of partial agonist beta blocker monotherapy for primary hypertension. Cochrane Database Syst. Rev. CD007450 (2014).
Lesch, K.-P. et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531 (1996).
Acknowledgements
Financial support was provided by NIH Awards GM46059 (S.L.B.), F32GM108294 (D.T.C.), F32GM122204 (L.H.), F30HD093358 (C.M.F.) and GM110535 (B.L.P.) and also by MIT startup funds, a Damon Runyon Cancer Research Foundation Award and a Sontag Distinguished Scientist Award for B.L.P. C.Z. is a recipient of a George Büchi Summer Research Fellowship, a Koch Graduate Fellowship in Cancer Research and a Bristol-Myers Squibb Fellowship in Synthetic Organic Chemistry. A.J.M. is a National Science Foundation Graduate Research Fellow. The authors acknowledge the Biological Instrument Facility of MIT for providing the Octet BioLayer Interferometry System (NIH S10 OD016326) and CD spectrometer (NSF-0070319). We thank Y.-M. Wang and M. Pirnot (MIT) for help in preparing this article. We also acknowledge S. Bano (Merck) for Cu ICP-MS analysis of the purified peptides 13c, 13d, 13e and 13f.
Author information
Authors and Affiliations
Contributions
D.T.C. and B.L.P. conceived the work and designed the experiments. D.T.C., C.Z. and C.M.F. performed the peptide and protein labelling experiments. D.T.C., C.M.F. and A.J.M. developed the seleno–affibody synthesis. L.H. and S.J.M. performed the NMR characterization to assign regioselectivity for the selenocysteine conjugation. K.D.J., Z.S. and O.P. carried out the cytotoxicity and haemolysis assays on 23v analogues. D.T.C., C.Z., C.M.F. and B.L.P. wrote the manuscript, with input from all other authors.
Corresponding authors
Ethics declarations
Competing interests
K.D.J., Z.S. and O.P. are employees of Visterra Inc. D.T.C., C.Z., S.L.B. and B.L.P. are inventors on a patent filed by MIT-TLO to cover this work (US patent application no. 15,187,169 and international application (PCT/US16/38372).
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Materials, Methods, and References
Rights and permissions
About this article
Cite this article
Cohen, D.T., Zhang, C., Fadzen, C.M. et al. A chemoselective strategy for late-stage functionalization of complex small molecules with polypeptides and proteins. Nature Chem 11, 78–85 (2019). https://doi.org/10.1038/s41557-018-0154-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0154-0
This article is cited by
-
Selenium chemistry for spatio-selective peptide and protein functionalization
Nature Reviews Chemistry (2024)
-
Arylglycine: A Focus on Amino Acid Preparation and Peptide Synthesis
International Journal of Peptide Research and Therapeutics (2022)
-
Site-selective photocatalytic functionalization of peptides and proteins at selenocysteine
Nature Communications (2022)
-
Effects of Water Content on Physicochemical Properties of a Protic Ionic Liquid with Monoprotic N-Hexylethylenediaminium as Cation and Bis(trifluoromethylsulfonyl) Imide as Anion
Journal of Solution Chemistry (2021)
-
Single electron transfer-based peptide/protein bioconjugations driven by biocompatible energy input
Communications Chemistry (2020)