Abstract
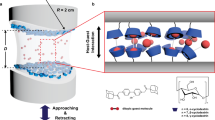
The accurate dissection of binding energies into their microscopic components is challenging, especially in solution. Here we study the binding of noble gases (He–Xe) with the macrocyclic receptor cucurbit[5]uril in water by displacement of methane and ethane as 1H NMR probes. We dissect the hydration free energies of the noble gases into an attractive dispersive component and a repulsive one for formation of a cavity in water. This allows us to identify the contributions to host–guest binding and to conclude that the binding process is driven by differential cavitation energies rather than dispersion interactions. The free energy required to create a cavity to accept the noble gas inside the cucurbit[5]uril is much lower than that to create a similarly sized cavity in bulk water. The recovery of the latter cavitation energy drives the overall process, which has implications for the refinement of gas-storage materials and the understanding of biological receptors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information and from the corresponding authors upon reasonable request.
Change history
16 October 2018
In the version of this Article originally published online, Fig. 2e was missing its data points; this has now been corrected in all versions of the Article.
References
Shabtai, E. et al. 3He NMR of He@C60 6− and He@C70 6 −. New records for the most shielded and the most deshielded 3He inside a fullerene. J. Am. Chem. Soc. 120, 6389–6393 (1998).
Tilton, R. F., Kuntz, I. D. & Petsko, G. A. Cavities in proteins: structure of a metmyoglobin xenon complex solved to 1.9 Å. Biochemistry 23, 2849–2857 (1984).
Hill, P. A., Wei, Q., Eckenhoff, R. G. & Dmochowski, I. J. Thermodynamics of xenon binding to cryptophane in water and human plasma. J. Am. Chem. Soc. 129, 9262–9263 (2007).
Jacobson, D. R. et al. Measurement of radon and xenon binding to a cryptophane molecular host. Proc. Natl Acad. Sci. USA 108, 10969–10973 (2011).
Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem. Eur. J. 18, 9955–9964 (2012).
Duignan, T. T., Parsons, D. F. & Ninham, B. W. A continuum solvent model of the multipolar dispersion solvation energy. J. Phys. Chem. B 117, 9412–9420 (2013).
Duignan, T. T., Parsons, D. F. & Ninham, B. W. A continuum model of solvation energies including electrostatic, dispersion, and cavity contributions. J. Phys. Chem. B 117, 9421–9429 (2013).
Muddana, H. S., Fenley, A. T., Mobley, D. L. & Gilson, M. K. The SAMPL4 host–guest blind prediction challenge: an overview. J. Comput. Aided Mol. Des. 28, 305–317 (2014).
Henriksen, N. M., Fenley, A. T. & Gilson, M. K. Computational calorimetry: high-precision calculation of host–guest binding thermodynamics. J. Chem. Theory Comput. 11, 4377–4394 (2015).
Sure, R. & Grimme, S. Comprehensive benchmark of association (free) energies of realistic host–guest complexes. J. Chem. Theory Comput. 11, 3785–3801 (2015).
Hostaš, J. et al. A nexus between theory and experiment: non-empirical quantum mechanical computational methodology applied to cucurbit[n]uril⋅guest binding interactions. Chem. Eur. J. 22, 17226–17238 (2016).
Hunter, C. A. Quantifying intermolecular interactions: guidelines for the molecular recognition toolbox. Angew. Chem. Int. Ed. 43, 5310–5324 (2004).
Yang, L., Adam, C., Nichol, G. S. & Cockroft, S. L. How much do van der Waals dispersion forces contribute to molecular recognition in solution? Nat. Chem. 5, 1006–1010 (2013).
Shimizu, K. D. Intermolecular forces: a solution to dispersion interactions. Nat. Chem. 5, 989–990 (2013).
Adam, C., Yang, L. & Cockroft, S. L. Partitioning solvophobic and dispersion forces in alkyl and perfluoroalkyl cohesion. Angew. Chem. Int. Ed. 54, 1164–1167 (2015).
Yang, L., Adam, C. & Cockroft, S. L. Quantifying solvophobic effects in nonpolar cohesive interactions. J. Am. Chem. Soc. 137, 10084–10087 (2015).
Hwang, J. et al. How important are dispersion interactions to the strength of aromatic stacking interactions in solution? Chem. Sci. 6, 4358–4364 (2015).
Yang, L., Brazier, J. B., Hubbard, T. A., Rogers, D. M. & Cockroft, S. L. Can dispersion forces govern aromatic stacking in an organic solvent? Angew. Chem. Int. Ed. 55, 912–916 (2016).
Hwang, J., Li, P., Smith, M. D. & Shimizu, K. D. Distance‐dependent attractive and repulsive interactions of bulky alkyl groups. Angew. Chem. Int. Ed. 55, 8086–8089 (2016).
Schneider, H.-J. Dispersive interactions in solution complexes. Acc. Chem. Res. 48, 1815–1822 (2015).
Wagner, J. P. & Schreiner, P. R. London dispersion in molecular chemistry—reconsidering steric effects. Angew. Chem. Int. Ed. 54, 12274–12296 (2015).
Barrow, S. J., Kasera, S., Rowland, M. J., del Barrio, J. & Scherman, O. A. Cucurbituril-based molecular recognition. Chem. Rev. 115, 12320–12406 (2015).
Nau, W. M., Florea, M. & Assaf, K. I. Deep inside cucurbiturils: physical properties and volumes of their inner cavity determine the hydrophobic driving force for host–guest complexation. Isr. J. Chem. 51, 559–577 (2011).
Lagona, J., Mukhopadhyay, P., Chakrabarti, S. & Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Ed. 44, 4844–4870 (2005).
Kim, J. et al. New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 122, 540–541 (2000).
Miyahara, Y., Abe, K. & Inazu, T. ‘Molecular’ molecular sieves: lid-free decamethylcucurbit[5]uril absorbs and desorbs gases selectively. Angew. Chem. Int. Ed. 41, 3020–3023 (2002).
Nguyen, B. T. & Anslyn, E. V. Indicator-displacement assays. Coord. Chem. Rev. 250, 3118–3127 (2006).
Assaf, K. I. & Nau, W. M. Cucurbiturils: from synthesis to high-affinity binding and catalysis. Chem. Soc. Rev. 44, 394–418 (2015).
Florea, M. & Nau, W. M. Strong binding of hydrocarbons to cucurbituril probed by fluorescent dye displacement: a supramolecular gas-sensing ensemble. Angew. Chem. Int. Ed. 50, 9338–9342 (2011).
Kumar, C. P., Wu, F., Woodward, C. E. & Day, A. I. The influence of equatorial substitution and K+ ion concentration: an encapsulation study of CH4, CH3F, CH3Cl, CH2F2 and CF4, in Q[5], CyP5Q[5] and a CyP5Q[5]-carboxylate derivative. Supramol. Chem. 26, 670–676 (2014).
Ustrnul, L., Kulhanek, P., Lizal, T. & Sindelar, V. Pressocucurbit[5]uril. Org. Lett. 17, 1022–1025 (2015).
Mecozzi, S. & Rebek, J. The 55% solution: a formula for molecular recognition in the liquid state. Chem. Eur. J. 4, 1016–1022 (1998).
Qvist, J., Davidovic, M., Hamelberg, D. & Halle, B. A dry ligand-binding cavity in a solvated protein. Proc. Natl Acad. Sci. USA 105, 6296–6301 (2008).
Rosenzweig, A. C., Frederick, C. A., Lippard, S. J. & Nordlund, P. Crystal structure of a bacterial non-haem iron hydroxylase that catalyses the biological oxidation of methane. Nature 366, 537–543 (1993).
Quillin, M. L., Breyer, W. A., Griswold, I. J. & Matthews, B. W. Size versus polarizability in protein–ligand interactions: binding of noble gases within engineered cavities in phage T4 lysozyme1. J. Mol. Biol. 302, 955–977 (2000).
Yang, C. et al. Fluorous metal–organic frameworks with superior adsorption and hydrophobic properties toward oil spill cleanup and hydrocarbon storage. J. Am. Chem. Soc. 133, 18094–18097 (2011).
Biedermann, F., Nau, W. M. & Schneider, H.-J. The hydrophobic effect revisited—studies with supramolecular complexes imply high-energy water as a noncovalent driving force. Angew. Chem. Int. Ed. 53, 11158–11171 (2014).
Chandler, D. Interfaces and the driving force of hydrophobic assembly. Nature 437, 640–647 (2005).
Abraham, M. H., Whiting, G. S., Fuchs, R. & Chambers, E. J. Thermodynamics of solute transfer from water to hexadecane. J. Chem. Soc. Perkin Trans. 2, 291–300 (1990).
Assaf, K. I. & Nau, W. M. Cucurbiturils as fluorophilic receptors. Supramol. Chem. 26, 657–669 (2014).
Otto, S. The role of solvent cohesion in nonpolar solvation. Chem. Sci. 4, 2953–2959 (2013).
Biedermann, F., Uzunova, V. D., Scherman, O. A., Nau, W. M. & De Simone, A. Release of high-energy water as an essential driving force for the high-affinity binding of cucurbit[n]urils. J. Am. Chem. Soc. 134, 15318–15323 (2012).
Nguyen, C. N., Young, T. K. & Gilson, M. K. Grid inhomogeneous solvation theory: hydration structure and thermodynamics of the miniature receptor cucurbit[7]uril. J. Chem. Phys. 137, 044101 (2012).
Genheden, S., Kongsted, J., Söderhjelm, P. & Ryde, U. Nonpolar solvation free energies of protein–ligand complexes. J. Chem. Theory Comput. 6, 3558–3568 (2010).
Sheeba Jem, I. & Richard, H. H. Solvation theory to provide a molecular interpretation of the hydrophobic entropy loss of noble-gas hydration. J. Phys. Condens. Matter 22, 284108 (2010).
Liu, T. & Schneider, H.-J. Additivity and quantification of dispersive interactions—from cyclopropyl to nitro groups: measurements on porphyrin derivatives. Angew. Chem. Int. Ed. 41, 1368–1370 (2002).
Marquez, C. & Nau, W. M. Polarizabilities inside molecular containers. Angew. Chem. Int. Ed. 40, 4387–4390 (2001).
Campanell, F. C., Battino, R. & Seybold, P. G. On the role of solute polarizability in determining the solubilities of gases in liquids. J. Chem. Eng. Data 55, 37–40 (2010).
Ivanov, E. V., Lebedeva, E. J., Abrosimov, V. K. & Ivanova, N. G. Structural contribution of the effect of hydrophobic hydration of noble gases. J. Struct. Chem. 46, 253–263 (2005).
Wilhelm, E., Battino, B. & Wilcock, R. J. Low-pressure solubility of gases in liquid water. Chem. Rev. 77, 219–262 (1977).
Acknowledgements
W.M.N. thanks A. Ben-Naim for helpful discussions, A. Barba-Bon for control experiments and the DFG (grant no. NA 681/8 within the SPP 1807 ‘Control of London dispersion interactions in molecular chemistry’) for financial support. N.V. thanks A. Mavrantonakis for helpful discussions. S.H. acknowledges support from the China Scholarship Council. F.B. thanks the DFG Emmy Noether programme (BI 1805/2-1). T.T.D. thanks D. Parsons for helpful discussions and acknowledges support from the US Department of Energy, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences.
Author information
Authors and Affiliations
Contributions
F.B. initiated this project with W.M.N. The manuscript was written by S.H., F.B. and W.M.N., and commented on by all authors. All gas binding experiments by 1H NMR were conducted by S.H. and F.B. in the laboratories of W.M.N. 3He NMR experiments were carried out by R.E.H. and water-suppression NMR experiments were carried out by A.D.S. Quantum-chemical calculations were carried out by N.V., L.Z. and T.H. and CSM–D calculations by T.T.D.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary Information
Experimental section; Supplementary computations; Supplementary Figures 1–10
Rights and permissions
About this article
Cite this article
He, S., Biedermann, F., Vankova, N. et al. Cavitation energies can outperform dispersion interactions. Nature Chem 10, 1252–1257 (2018). https://doi.org/10.1038/s41557-018-0146-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0146-0
This article is cited by
-
Unveiling the gemcitabine drug complexation with cucurbit[n]urils (n = 6–8): a computational analysis
Structural Chemistry (2023)
-
Cucurbit[n]urils (n = 7, 8) can strongly bind neutral hydrophilic molecules in water
Science China Chemistry (2022)
-
Xenon binding by a tight yet adaptive chiral soft capsule
Nature Communications (2020)
-
Cucurbiturils in supramolecular catalysis
Journal of Inclusion Phenomena and Macrocyclic Chemistry (2020)
-
Polarizability and isotope effects on dispersion interactions in water
Communications Chemistry (2019)