Abstract
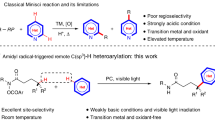
Chemists’ ability to synthesize structurally complex, high-value organic molecules from simple starting materials is limited by methods to selectively activate and functionalize strong alkyl C(sp3) covalent bonds. Recent activity has focused on the activation of abundant C–O, C–N and C–C bonds via a mechanistic paradigm of oxidative addition of a low-valent, electron-rich transition metal. This approach typically employs nickel(0), rhodium(i), ruthenium(0) and iron catalysts under conditions finely tuned for specific, electronically activated substrates, sometimes assisted by chelating functional groups or ring strain. By adopting a redox-neutral strategy involving palladium(ii)-catalysed C–H activation followed by β-heteroatom/carbon elimination, we describe here a catalytic method to activate alkyl C(sp3)–oxygen, nitrogen, carbon, fluorine and sulfur bonds with high regioselectivity. Directed hydrofunctionalization of the resultant palladium(ii)-bound alkene leads to formal functional group metathesis. The method is applied to amino acid upgrading with complete regioselectivity and moderate to high retention of enantiomeric excess. Low-strain heterocycles undergo strong-bond activation and substitution, giving ring-opened products.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Yu, D.-G., Li, B.-J. & Shi, Z.-J. Exploration of new C–O electrophiles in cross-coupling reactions. Acc. Chem. Res. 43, 1486–1495 (2010).
Rosen, B. M. et al. Nickel-catalyzed cross-coupling involving carbon–oxygen bonds. Chem. Rev. 111, 1346–1416 (2011).
Cornella, J., Zarate, C. & Martin, R. Metal-catalyzed activation of ethers via C–O bond cleavage: a new strategy for molecular diversity. Chem. Soc. Rev. 43, 8081–8097 (2014).
Dermenci, A., Coe, J. W. & Dong, G. Direct activation of relatively unstrained carbon–carbon bonds in homogeneous systems. Org. Chem. Front. 1, 567–581 (2014).
Huang, C.-Y. & Doyle, A. G. The chemistry of transition metals with three-membered ring heterocycles. Chem. Rev. 114, 8153–8198 (2014).
Tobisu, M. & Chatani, N. Cross-couplings using aryl ethers via C–O bond activation enabled by nickel catalysts. Acc. Chem. Res. 48, 1717–1726 (2015).
Tobisu, M. & Chatani, N. Nickel-catalyzed cross-coupling reactions of unreactive phenolic electrophiles via C–O bond activation. Top. Curr. Chem. 374, 41 (2016).
Zarate, C., van Gemmeren, M., Somerville, R. J. & Martin, R. Phenol derivatives: modern electrophiles in cross-coupling reactions. Adv. Organomet. Chem. 66, 143–222 (2016).
Chen, P.-H., Billett, B. A., Tsukamoto, T. & Dong, G. ‘Cut and sew’ transformations via transition-metal-catalyzed carbon–carbon bond activation. ACS Catal. 7, 1340–1360 (2017).
Dander, J. E. & Garg, N. K. Breaking amides using nickel catalysis. ACS Catal. 7, 1413–1423 (2017).
Matsunaga, P. T. & Hillhouse, G. L. Thianickelacycles by ring-opening reactions of cyclic thioethers and their subsequent carbonylation to thioesters. Angew. Chem. Int. Ed. 33, 1748–1749 (1994).
Lin, B. L., Clough, C. R. & Hillhouse, G. L. Interactions of aziridines with nickel complexes: oxidative-addition and reductive-elimination reactions that break and make C–N bonds. J. Am. Chem. Soc. 124, 2890–2891 (2002).
Dankwardt, J. W. Nickel-catalyzed cross-coupling of aryl Grignard reagents with aromatic alkyl ethers: an efficient synthesis of unsymmetrical biaryls. Angew. Chem. Int. Ed. 43, 2428–2432 (2004).
Ueno, S., Mizushima, E., Chatani, N. & Kakiuchi, F. Direct observation of the oxidative addition of the aryl carbon–oxygen bond to a ruthenium complex and consideration of the relative reactivity between aryl carbon–oxygen and aryl carbon–hydrogen bonds. J. Am. Chem. Soc. 128, 16516–16517 (2006).
Quasdorf, K. W., Riener, M., Petrova, K. V. & Garg, N. K. Suzuki–Miyaura coupling of aryl carbamates, carbonates, and sulfamates. J. Am. Chem. Soc. 131, 17748–17749 (2009).
Barbero, N. & Martin, R. Ligand-free Ni-catalyzed reductive cleavage of inert carbon–sulfur bonds. Org. Lett. 14, 796–799 (2012).
Sergeev, A. G., Webb, J. D. & Hartwig, J. F. A heterogeneous nickel catalyst for the hydrogenolysis of aryl ethers without arene hydrogenation. J. Am. Chem. Soc. 134, 20226–20229 (2012).
Juliá-Hernández, F., Ziadi, A., Nishimura, A. & Martin, R. Nickel-catalyzed chemo-, regio- and diastereoselective bond formation through proximal C–C cleavage of benzocyclobutenones. Angew. Chem. Int. Ed. 54, 9537–9541 (2015).
Liu, X.-W., Echavarren, J., Zarate, C. & Martin, R. Ni-catalyzed borylation of aryl fluorides via C–F cleavage. J. Am. Chem. Soc. 137, 12470–12473 (2015).
Hie, L. et al. Nickel-catalyzed activation of acyl C–O bonds of methyl esters. Angew. Chem. Int. Ed. 55, 2810–2814 (2016).
Moragas, T., Gaydou, M. & Martin, R. Nickel-catalyzed carboxylation of benzylic C–N bonds with CO2. Angew. Chem. Int. Ed. 55, 5053–5057 (2016).
van der Boom, M. E., Liou, S.-Y., Ben-David, Y., Shimon, L. J. W. & Milstein, D. Alkyl– and aryl–oxygen bond activation in solution by rhodium(i), palladium(ii), and nickel(ii). Transition-metal-based selectivity. J. Am. Chem. Soc. 120, 6531–6541 (1998).
Lara, P. et al. Formation and cleavage of C–H, C–C, and C–O bonds of ortho-methyl-substituted anisoles by late transition metals. J. Am. Chem. Soc. 128, 3512–3513 (2006).
Fulmer, G. R., Muller, R. P., Kemp, R. A. & Goldberg, K. I. Hydrogenolysis of palladium(ii) hydroxide and methoxide pincer complexes. J. Am. Chem. Soc. 131, 1346–1347 (2009).
Santos, L. L., Mereiter, K. & Paneque, M. Reaction of 2-methylanisole with TpMe2Ir(C6H5)2(N2): a comprehensive set of activations. Organometallics 32, 565–569 (2013).
Edouard, G. A., Kelley, P., Herbert, D. E. & Agapie, T. Aryl ether cleavage by group 9 and 10 transition metals: stoichiometric studies of selectivity and mechanism. Organometallics 34, 5254–5277 (2015).
Chu, T., Boyko, Y., Korobkov, I. & Nikonov, G. I. Transition metal-like oxidative addition of C–F and C–O bonds to an aluminum(i) center. Organometallics 34, 5363–5365 (2015).
Crimmin, M. R., Butler, M. J. & White, A. J. P. Oxidative addition of carbon–fluorine and carbon–oxygen bonds to Al(i). Chem. Commun. 51, 15994–15996 (2015).
Henry, P. M. Palladium(ii)-catalyzed exchange and isomerization reactions. IV. Exchange of vinylic chloride with radioactive lithium chloride catalyzed by palladium(ii) chloride in acetic acid. J. Org. Chem. 37, 2443–2447 (1972).
Henry, P. M. Palladium(ii)-catalyzed exchange and isomerization reactions. Acc. Chem. Res. 6, 16–24 (1973).
Catellani, M. & Fagnola, M. C. Palladacycles as intermediates for selective dialkylation of arenes and subsequent fragmentation. Angew. Chem. Int. Ed. 33, 2421–2422 (1995).
Zhao, H., Ariafard, A. & Lin, Z. β-Heteroatom versus β-hydrogen elimination: a theoretical study. Organometallics 25, 812–819 (2006).
Yang, J., Mercer, G. J. & Nguyen, H. M. Palladium-catalyzed glycal imidate rearrangement: formation of α- and β-N-glycosyl trichloroacetamides. Org. Lett. 9, 4231–4234 (2007).
White, P. B. & Stahl, S. S. Reversible alkene insertion into the Pd–N bond of Pd(ii)-sulfonamidates and implications for catalytic amidation reactions. J. Am. Chem. Soc. 133, 18594–18597 (2011).
Jana, R., Pathak, T. P., Jensen, K. H. & Sigman, M. S. Palladium(ii)-catalyzed enantio- and diastereoselective synthesis of pyrrolidine derivatives. Org. Lett. 14, 4074–4077 (2012).
Choi, J. et al. Cleavage of sp 3 C–O bonds via oxidative addition of C–H bonds. J. Am. Chem. Soc. 131, 15627–15629 (2009).
Choi, J. et al. Net oxidative addition of C(sp 3)–F bonds to iridium via initial C–H bond activation. Science 332, 1545–1548 (2011).
Haibach, M. C., Lease, N. & Goldman, A. S. Catalytic cleavage of ether C–O bonds by pincer iridium complexes. Angew. Chem. Int. Ed. 53, 10160–10163 (2014).
Ogiwara, Y., Kochi, T. & Kakiuchi, F. Ruthenium-catalyzed conversion of sp 3 C–O bonds in ethers to C–C bonds using triarylboroxines. Org. Lett. 13, 3254–3257 (2011).
Luo, S. et al. Fe-promoted cross coupling of homobenzylic methyl ethers with Grignard reagents via sp 3 C–O bond cleavage. Chem. Commun. 49, 7794–7796 (2013).
Zaitsev, V. G., Shabashov, D. & Daugulis, O. Highly regioselective arylation of sp 3 C–H bonds catalyzed by palladium acetate. J. Am. Chem. Soc. 127, 13154–13155 (2005).
Gurak, J. A. Jr., Yang, K. S., Liu, Z. & Engle, K. M. Directed, regiocontrolled hydroamination of unactivated alkenes via protodepalladation. J. Am. Chem. Soc. 138, 5805–5808 (2016).
Yang, K., Gurak, J. A. Jr., Liu, Z. & Engle, K. M. Catalytic, regioselective hydrocarbofunctionalization of unactivated alkenes with diverse C–H nucleophiles. J. Am. Chem. Soc. 138, 14705–14712 (2016).
Gurak, J. A. Jr., Tran, V. T., Sroda, M. M. & Engle, K. M. N-alkylation of 2-pyridone derivatives via palladium(ii)-catalyzed directed alkene hydroamination. Tetrahedron 73, 3636–3642 (2017).
Wada, S. & Jordan, R. F. Olefin insertion into a Pd–F bond: catalyst reactivation following β-F elimination in ethylene/vinyl fluoride copolymerization. Angew. Chem. Int. Ed. 129, 1846–1850 (2017).
Ichikawa, J., Nadano, R., & Ito, N. 5-endo Heck-type cyclization of 2-(trifluoromethyl)allyl ketone oximes: synthesis of 4-difluoromethylene-substituted 1-pyrrolines. Chem. Commun 4425–4427 (2006).
Li, Y., Yang, W., Cheng, G. & Yang, D. Palladium-catalyzed syn-stereocontrolled ring-opening of oxabicyclic alkenes with sodium arylsulfinates. J. Org. Chem. 81, 4744–4750 (2016).
Acknowledgements
This work was financially supported by TSRI, Bristol-Myers Squibb (Unrestricted Grant), Pfizer, Inc., and the National Institutes of Health (1R35GM125052). We also gratefully acknowledge the following graduate fellowship programs: Frank J. Dixon Fellowship (V.T.T.), Donald E. and Delia B. Baxter Foundation (J.A.G.), and the National Science Foundation (NSF/DGE-1346837) (J.A.G.). We thank Dr. Jason S. Chen for determination of enantiomeric excess, along with Dr. Dee-Hua Huang and Dr. Laura Pasternack for assistance with NMR spectroscopy. We also thank Prof. Arnold L. Rheingold, Dr. Milan Gembicky, and Dr. Curtis E. Moore (UCSD) for X-ray crystallographic analysis. Prof. Jin-Quan Yu is thanked for helpful discussions.
Author information
Authors and Affiliations
Contributions
V.T.T., J.A.G., K.S.Y., and K.M.E. conceived this work. V.T.T. and J.A.G. performed the experiments and analyzed the data. V.T.T. and K.M.E. wrote the manuscript with input from J.A.G. and K.S.Y.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information
General information, optimization data, mechanistic studies, substrate synthesis, general procedures, additional references and characterization data
Crystallographic data
CIF for compound 1m’; CCDC reference: 1840316
Crystallographic data
CIF for compound S1; CCDC reference: 1840315
NMR data
Original NMR data in FID format
Rights and permissions
About this article
Cite this article
Tran, V.T., Gurak, J.A., Yang, K.S. et al. Activation of diverse carbon–heteroatom and carbon–carbon bonds via palladium(ii)-catalysed β-X elimination. Nature Chem 10, 1126–1133 (2018). https://doi.org/10.1038/s41557-018-0110-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0110-z
This article is cited by
-
Recent Progress in NiH-Catalyzed Linear or Branch Hydrofunctionalization of Terminal or Internal Alkenes
Topics in Current Chemistry (2023)
-
NiH-catalysed proximal-selective hydroalkylation of unactivated alkenes and the ligand effects on regioselectivity
Nature Communications (2022)
-
Divergent regioselective Heck-type reaction of unactivated alkenes and N-fluoro-sulfonamides
Nature Communications (2022)
-
Mechanistically informed selection rules for competing β-hydride and β-heteroatom eliminations
Nature Synthesis (2022)
-
Cobalt-catalyzed deoxygenative triborylation of allylic ethers to access 1,1,3-triborylalkanes
Nature Communications (2020)