Abstract
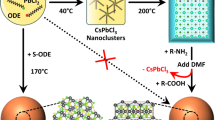
Biological and bio-inspired mineralization processes yield a variety of three-dimensional structures with relevance for fields such as photonics, electronics and photovoltaics. However, these processes are only compatible with specific material compositions, often carbonate salts, thereby hampering widespread applications. Here we present a strategy to convert a wide range of metal carbonate structures into lead halide perovskite semiconductors with tunable bandgaps, while preserving the 3D shape. First, we introduce lead ions by cation exchange. Second, we use carbonate as a leaving group, facilitating anion exchange with halide, which is followed rapidly by methylammonium insertion to form the perovskite. As proof of principle, pre-programmed carbonate salt shapes such as vases, coral-like forms and helices are transformed into perovskites while preserving the morphology and crystallinity of the initial micro-architectures. This approach also readily converts calcium carbonate biominerals into semiconductors, furnishing biological and programmable synthetic shapes with the performance of artificial compositions such as perovskite-based semiconductors.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Fratzl, P. Biomimetic materials research: what can we really learn from nature’s structural materials. J. R. Soc. Interface 4, 637–642 (2007).
Nudelman, F. & Sommerdijk, N. A. J. M. Biomineralization as an inspiration for materials chemistry. Angew. Chem. Int. Ed. 21, 6582–6596 (2012).
Studart, A. R. Towards high-performance bioinspired composites. Adv. Mater. 24, 5024–5044 (2012).
Wegst, U. G. K., Bai, H., Saiz, E., Tomsia, A. P. & Ritchie, R. O. Bioinspired structural materials. Nat. Mater. 14, 23–36 (2014).
Di Giosia, M. et al. Bioinspired nanocomposites: ordered 2D materials within a 3D lattice. Adv. Funct. Mater. 26, 5569–5575 (2016).
Xu, S. et al. Assembly of micro/nanomaterials into complex, three-dimensional architectures by compressive buckling. Science 347, 154–159 (2015).
Nie, Z., Petukhova, A. & Kumacheva, E. Properties and emerging applications of self-assembled structures made from inorganic nanoparticles. Nat. Nanotech. 5, 15–25 (2010).
Bao, Z. et al. Chemical reduction of three-dimensional silica micro-assemblies into microporous silicon replicas. Nature 446, 172–175 (2007).
Kaplan, C. N. et al. Controlled growth and form of precipitating microstructures. Science 355, 1395–1399 (2017).
Lowenstam, H. A. & Weiner, S. On Biomineralization (Oxford Univ. Press, Oxford, 1989).
Mann, S. Biomineralization (Oxford Univ. Press: Oxford, 2002).
Mann, S. & Ozin, G. A. Synthesis of inorganic materials with complex form. Nature 382, 313–318 (1996).
García-Ruiz, J. M., Melero-García, E. & Hyde, S. T. Morphogenesis of self-assembled nanocrystalline materials of barium carbonate and silica. Science 323, 362–365 (2009).
Noorduin, W. L., Grinthal, A., Mahadevan, L. & Aizenberg, J. Rationally designed complex, hierarchical microarchitectures. Science 340, 832–837 (2013).
Sandhage, K. H. et al. Novel, bioclastic route to self-assembled 3D, chemically tailored meso/nanostructures: shape-preserving reactive conversion of biosilica (diatom) microshells. Adv. Mater. 14, 429–433 (2002).
Weatherspoon, M. R., Allan, S. M., Hunt, E., Cai, Y. & Sandhage, K. H. Sol–gel synthesis on self-replicating single-cell scaffolds: applying complex chemistries to nature’s nanostructured templates. Chem. Commun. 2005, 651–653 (2005).
Wu, H. et al. Electrospun metal nanofiber webs as high-performance transparent electrodes. Nano Lett. 10, 4242–4248 (2010).
Son, D. H., Hughes, S. M., Yin, Y. & Alivisatos, A. P. Cation exchange reactions in ionic nanocrystals. Science 306, 1009–1012 (2004).
Robinson, R. D. et al. Spontaneous superlattice formation in nanorods through partial cation exchange. Science 317, 355–358 (2007).
Beberwyck, B. J., Surendranath, Y. & Alivisatos, A. P. Cation exchange: a versatile tool for nanomaterials synthesis. J. Phys. Chem. C 117, 19759–19770 (2013).
Putnis, A. Mineral replacement reactions: from macroscopic observations to microscopic mechanisms. Mineral. Mag. 66, 689–708 (2002).
De Trizio, L. & Manna, L. Forging colloidal nanostructures via cation exchange reactions. Chem. Rev. 116, 10852–10887 (2016).
Hodges, J. H., Kletetschka, K., Fenton, J. L., Read, C. G. & Schaak, R. E. Sequential anion and cation exchange reactions for complete material transformations of nanoparticles with morphological retention. Angew. Chem. Int. Ed. 54, 8669–8672 (2015).
Wu, H.-L. et al. Formation of pseudomorphic nanocages from Cu2O nanocrystals through anion exchange reactions. Science 351, 1306–1310 (2016).
Stebe, K. J., Lewandowski, E. & Ghosh, M. Oriented assembly of metamaterials. Science 325, 159–160 (2009).
Boneschanscher, M. P. et al. Long-range orientation and atomic attachment of nanocrystals in 2D honeycomb superlattices. Science 344, 1377–1380 (2014).
Geuchies, J. et al. In situ study of the formation mechanism of two-dimensional superlattices from PbSe nanocrystals. Nat. Mater. 15, 1248–1254 (2016).
Miszta, K. et al. Hierarchical self-assembly of suspended branched colloidal nanocrystals into superlattice structures. Nat. Mater. 10, 872–876 (2011).
Zhang, W., Eperon, G. E. & Snaith, H. J. Metal halide perovskites for energy applications. Nat. Energy 1, 16048–16056 (2016).
Polman, A. & Atwater, H. A. Photonic design principles for ultrahigh-efficiency photovoltaics. Nat. Mater. 11, 174–177 (2012).
Chen, K. & Tüysüz, H. Morphology-controlled synthesis of organometal halide perovskite inverse opals. Angew. Chem. Int. Ed. 54, 13806–13810 (2015).
Ashley, M. J. et al. Templated synthesis of uniform perovskite nanowire arrays. J. Am. Chem. Soc. 138, 10096–10099 (2016).
Burschka, J. et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature 499, 316–319 (2013).
Sheng, R. et al. Methylammonium lead bromide perovskite-based solar cells by vapor-assisted deposition. J. Phys. Chem. C 119, 3545–3549 (2015).
Yuan, K., Lee, S. S., De Andrade, V., Sturchio, N. C. & Fenter, P. Replacement of calcite (CaCO3) by cerussite (PbCO3). Environ. Sci. Technol. 50, 12984–12991 (2016).
Solubility Product Constants (North Carolina State University, accessed 1 May 2017); http://www4.ncsu.edu/~franzen/public_html/CH201/data/Solubility_Product_Constants.pdf
Niu, G., Li, W., Meng, F., Wang, L., Dong, H. & Qui, Y. Study on stability of CH3NH3PbI3 films and effect of post modification by aluminum oxide in all-solid-state hybrid solar cell. J. Mater. Chem. A 2, 705–711 (2014).
Tenuta, E., Zheng, C. & Rubel, O. Thermodynamic origin of instability in hybrid halide perovskites. Sci. Rep. 6, 37654 (2016).
Eperon, G. E. et al. The importance of moisture in hybrid lead halide perovskite thin film fabrication. ACS Nano 9, 9380–9393 (2015).
Li, L., Fijneman, A. J., Kaandorp, J. A., Aizenberg, J. & Noorduin, W. L. Directed nucleation and growth by balancing local supersaturation and substrate/nucleus lattice mismatch. Proc. Natl Acad. Sci. USA 115, 3575–3580 (2018).
Acknowledgements
The authors thank T. Coenen for assistance with the cathodoluminescence measurements, S. Brittman for technical help and discussions, L.M.C. Janssen for help with the manuscript and J.C. Weaver for identification of the biominerals. W.L.N. thanks the Netherlands Organization for Scientific Research (NWO) for financial support from a VENI grant. E.C.G. was partially supported by the European Research Council under the European Union’s Seventh Framework Programme (FP/2007–2013)/ERC grant agreement no. 337328, ‘NanoEnabledPV’ and by an STW VIDI grant. S.M. acknowledges funding from the European Research Council (grant agreement no. 695343). Scanning electron microscopy was performed at the fabrication and characterization facilities of the Amsterdam nanoCenter, supported by NWO.
Author information
Authors and Affiliations
Contributions
T.H., L.H. and H.C.H. contributed equally to this work. E.C.G. and W.L.N. conceived the initial idea. T.H., L.H. and H.C.H. designed, performed and analysed the experiments. I.B. designed and performed the methylamine detection. S.M. performed the cathodoluminescence analysis, and G.W.P.A. performed the photoluminescence lifetime measurements. W.L.N. wrote the manuscript, with input of all the other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary synthesis and characterization details
Supplementary Movie 1
Real-time movie of the conversion of a 3.5 cm sized sand dollar into CH3NH3PbBr3 by dripping a CH3NH3Br solution on the PbCO3 converted sand dollar surface under 365-nm UV illumination
Rights and permissions
About this article
Cite this article
Holtus, T., Helmbrecht, L., Hendrikse, H.C. et al. Shape-preserving transformation of carbonate minerals into lead halide perovskite semiconductors based on ion exchange/insertion reactions. Nature Chem 10, 740–745 (2018). https://doi.org/10.1038/s41557-018-0064-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0064-1
This article is cited by
-
Nature-inspired materials: Emerging trends and prospects
NPG Asia Materials (2021)
-
Broadband highly directive 3D nanophotonic lenses
Nature Communications (2018)
-
Thermal assisted self-organization of calcium carbonate
Nature Communications (2018)
-
Inorganic semiconductor biointerfaces
Nature Reviews Materials (2018)