Abstract
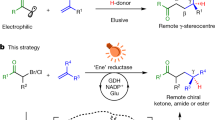
Strategies that provide enzymes with the ability to catalyse non-natural reactions are of considerable synthetic value. Photoredox catalysis has proved adept at expanding the synthetic repertoire of existing catalytic platforms, yet, in the realm of biocatalysis it has primarily been used for cofactor regeneration. Here we show that photoredox catalysts can be used to enable new catalytic function in nicotinamide-dependent enzymes. Under visible-light irradiation, xanthene-based photocatalysts enable a double-bond reductase to catalyse an enantioselective deacetoxylation. Mechanistic experiments support the intermediacy of an α-acyl radical, formed after the elimination of acetate. Isotopic labelling experiments support nicotinamide as the source of the hydrogen atom. Preliminary calculations and mechanistic experiments suggest that binding to the protein attenuates the reduction potential of the starting material, an important feature for localizing radical formation to the enzyme active site. The generality of this approach is highlighted with the radical dehalogenation of α-bromoamides catalysed by ketoreductases with Eosin Y as a photocatalyst.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Liang, J. et al. Highly enantioselective reduction of a small heterocyclic ketone: biocatalytic reduction of tetrahydrothiophene-3-one to the corresponding (R)-alcohol. Org. Process Res. Dev. 14, 188–192 (2010).
Bornscheuer, U. T. et al. Engineering the third wave of biocatalysis. Nature 485, 185–194 (2012).
Lin, C.-I., McCarty, R. M. & Liu, H.-W. The enzymology of organic transformations: a survey of name reactions in biological systems. Angew. Chem. Int. Ed. 56, 3446–3489 (2017).
Brandenberg, O. F., Fasan, R. & Arnold, F. H. Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions. Curr. Opin. Biotechnol. 47, 102–111 (2017).
Schwizer, F. et al. Artificial metalloenzymes: reaction scope and optimization strategies. Chem. Rev. 118, 142–231 (2018).
Prier, C. K. & Arnold, F. H. Chemomimetic biocatalysis: exploiting the synthetic potential of cofactor-dependent enzymes to create new catalysts. J. Am. Chem. Soc. 137, 13992–14006 (2015).
Bornscheuer, U. T. & Kazlauskas, R. J. Catalytic promiscuity in biocatalysis: using old enzymes to form new bonds and follow new pathways. Angew. Chem. Int. Ed. 43, 6032–6040 (2004).
Humble, M. S. & Berglund, P. Biocatalytic promiscuity. Eur. J. Org. Chem. 2011, 3391–3401 (2011).
Sibi, M. P., Manyem, S. & Zimmerman, J. Enantioselective radical processes. Chem. Rev. 103, 3263–3296 (2003).
Meggers, E. Asymmetric catalysis activated by visible light. Chem. Commun. 51, 3290–3301 (2015).
Yoon, T. P. Photochemical stereocontrol using tandem photoredox–chiral Lewis acid catalysis. Acc. Chem. Res. 49, 2307–2315 (2016).
Zhang, L. & Meggers, E. Steering asymmetric Lewis acid catalysis exclusively with octahedral metal-centered chirality. Acc. Chem. Res. 50, 320–330 (2017).
Skubi, K. L., Blum, T. R. & Yoon, T. P. Dual catalysis strategies in photochemical synthesis. Chem. Rev. 116, 10035–10074 (2016).
Nicewicz, D. A. & MacMillan, D. Merging photoredox catalysis with organocatalysis: the direct asymmetric alkylation of aldehydes. Science 322, 77 (2008).
Rono, L. J., Yayla, H. G., Wang, D. Y., Armstrong, M. F. & Knowles, R. R. Enantioselective photoredox catalysis enabled by proton-coupled electron transfer: development of an asymmetric aza-pinacol cyclization. J. Am. Chem. Soc. 135, 17735–17738 (2013).
Du, J., Skubi, K. L., Schultz, D. M. & Yoon, T. P. A dual-catalysis approach to enantioselective [2+2] photocycloadditions using visible light. Science 344, 392–396 (2014).
Huo, H. et al. Asymmetric photoredox transition-metal catalysis activated by visible light. Nature 515, 100–103 (2014).
Uraguchi, D., Kinoshita, N., Kizu, T. & Ooi, T. Synergistic catalysis of ionic Brønsted acid and photosensitizer for a redox neutral asymmetric α-coupling of N-arylaminomethanes with aldimines. J. Am. Chem. Soc. 137, 13768–13771 (2015).
Emmanuel, M. A., Greenberg, N. R., Oblinsky, D. G. & Hyster, T. K. Accessing non-natural reactivity by irradiating nicotinamide-dependent enzymes with light. Nature 540, 414–417 (2016).
Sandoval, B. A., Meichan, A. J. & Hyster, T. K. Enantioselective hydrogen atom transfer: discovery of catalytic promiscuity in flavin-dependent ‘ene’-reductases. J. Am. Chem. Soc. 139, 11313–11316 (2017).
Roberts, B. P. Polarity-reversal catalysis of hydrogen-atom abstraction reactions: concepts and applications in organic chemistry. Chem. Soc. Rev. 28, 25–35 (1999).
Romero, N. A. & Nicewicz, D. A. Organic photoredox catalysis. Chem. Rev. 116, 10075–10166 (2016).
Prier, C. K., Rankic, D. A. & MacMillan, D. W. C. Visible light photoredox catalysis with transition metal complexes: applications in organic synthesis. Chem. Rev. 113, 5322–5363 (2013).
Maciá-Agulló, J. A., Corma, A. & Garcia, H. Photobiocatalysis: the power of combining photocatalysis and enzymes. Chem. Eur. J. 21, 10940–10959 (2015).
Hollmann, F., Taglieber, A., Schulz, F. & Reetz, M. T. A light-driven stereoselective biocatalytic oxidation. Angew. Chem. Int. Ed. 46, 2903–2906 (2007).
Lee, S. H. et al. Cofactor-free, direct photoactivation of enoate reductases for the asymmetric reduction of C=C bonds. Angew. Chem. Int. Ed. 56, 8581–8685 (2017).
Mifsud, M. et al. Photobiocatalytic chemistry of oxidoreductases using water as the electron donor. Nat. Commun. 5, 3145 (2014).
Park, J. H. et al. Cofactor-free light-driven whole-cell cytochrome P450 catalysis. Angew. Chem. Int. Ed. 54, 969–973 (2014).
Tran, N.-H. et al. An efficient light-driven P450 BM3 biocatalyst. J. Am. Chem. Soc. 135, 14484–14487 (2013).
Brown, K. A., Wilker, M. B., Boehm, M., Dukovic, G. & King, P. W. Characterization of photochemical processes for H2 production by CdS nanorod–[FeFe] hydrogenase complexes. J. Am. Chem. Soc. 134, 5627–5636 (2012).
Onoda, A., Kihara, Y., Fukumoto, K., Sano, Y. & Hayashi, T. Photoinduced hydrogen evolution catalyzed by a synthetic diiron dithiolate complex embedded within a protein matrix. ACS Catal. 4, 2645–2648 (2014).
Brown, K. A. et al. Light-driven dinitrogen reduction catalyzed by a CdS:nitrogenase MoFe protein biohybrid. Science 352, 448–450 (2016).
Lazarides, T. et al. Visible light-driven O2 reduction by a porphyrin–laccase system. J. Am. Chem. Soc. 135, 3095–3103 (2013).
Gu, Y., Ellis-Guardiola, K., Srivastava, P. & Lewis, J. C. Preparation, characterization, and oxygenase activity of a photocatalytic artificial enzyme. ChemBioChem 16, 1880–1883 (2015).
O’Farrell, P. A., Walsh, M. A., McCarthy, A. A. & Higgins, T. M. Modulation of the redox potentials of FMN in Desulfovibrio vulgaris flavodoxin: thermodynamic properties and crystal structures of glycine-61 mutants. Biochemistry 37, 8405–8416 (1998).
Olea, C. Jr, Kuriyan, J. & Marletta, M. A. Modulating heme redox potential through protein-induced porphyrin distortion. J. Am. Chem. Soc. 132, 12794–12795 (2010).
Lin, F., Fan, W. & Wise, G. E. Eosin Y staining of proteins in polyacrylamide gels. Anal. Biochem. 196, 279–283 (1991).
Zhang, Y. & Görner, H. Photoprocesses of xanthene dyes bound to lysozyme or serum albumin. Photochem. Photobiol. 85, 677–685 (2009).
Banerjee, A., Lee, K., Yu, Q., Fang, A. G. & Falvey, D. E. Protecting group release through photoinduced electron transfer: wavelength control through sensitized irradiation. Tetrahedron Lett. 39, 4635–4638 (1998).
Monos, T. M., Magallanes, G., Sebren, L. J. & Stephenson, C. R. J. Visible light mediated reductions of ethers, amines and sulfides. J. Photochem. Photobiol. A 328, 240–248 (2016).
Tarantino, K. T., Liu, P. & Knowles, R. R. Catalytic ketyl–olefin cyclizations enabled by proton-coupled electron transfer. J. Am. Chem. Soc. 135, 10022–10025 (2013).
Huang, M. et al. Carbon–carbon double-bond reductases in nature. Drug Metab. Rev. 46, 362–378 (2014).
Youn, B. et al. Mechanistic and structural studies of apoform, binary, and ternary complexes of the arabidopsis alkenal double bond reductase At5g16970. J. Biol. Chem. 281, 40076–40088 (2006).
Mohr, J. T., Hong, A. Y. & Stoltz, B. M. Enantioselective protonation. Nat. Chem. 1, 359–369 (2009).
Mansell, D. J. et al. Biocatalytic asymmetric alkene reduction: crystal structure and characterization of a double bond reductase from Nicotiana tabacum. ACS Catal. 3, 370–379 (2013).
Lambert, C. R. & Kochevar, I. E. Electron transfer quenching of the Rose Bengal triplet state. Photochem. Photobiol. 66, 15–25 (1997).
Tanaka, M., Ohkubo, K. & Fukuzumi, S. DNA cleavage by UVA irradiation of NADH with dioxygen via radical chain processes. J. Phys. Chem. A 110, 11214–11218 (2006).
Yayla, H. G. et al. Discovery and mechanistic study of a photocatalytic indoline dehydrogenation for the synthesis of elbasvir. Chem. Sci. 7, 2066–2073 (2016).
Acknowledgements
This work is supported by Princeton University and a Searle Scholar Award (SSP-2017-1741) to T.K.H. We thank the MacMillan group for use of their Chiral HPLC and CV equipment. We thank C. Leahy for assistance in protein expression and purification.
Author information
Authors and Affiliations
Contributions
K.F.B. and S.J.C. contributed equally. K.F.B., S.J.C., M.A.E. and T.K.H. designed the experiments. K.F.B., S.J.C. and M.A.E. performed and analysed experiments. D.C.M. conducted the density functional theory calculations. T.K.H. prepared the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods and Procedures, Supplementary Sequence Information, Supplementary Data and Supplementary Figures
Rights and permissions
About this article
Cite this article
Biegasiewicz, K.F., Cooper, S.J., Emmanuel, M.A. et al. Catalytic promiscuity enabled by photoredox catalysis in nicotinamide-dependent oxidoreductases. Nature Chem 10, 770–775 (2018). https://doi.org/10.1038/s41557-018-0059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0059-y
This article is cited by
-
Towards the rate limit of heterologous biotechnological reactions in recombinant cyanobacteria
Biotechnology for Biofuels and Bioproducts (2023)
-
Photoenzymatic enantioselective intermolecular radical hydroamination
Nature Catalysis (2023)
-
Using enzymes to tame nitrogen-centred radicals for enantioselective hydroamination
Nature Chemistry (2023)
-
Enantioselective decarboxylative alkylation using synergistic photoenzymatic catalysis
Nature Catalysis (2023)
-
Photoinduced chemomimetic biocatalysis for enantioselective intermolecular radical conjugate addition
Nature Catalysis (2022)