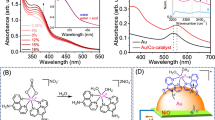
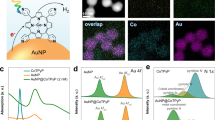
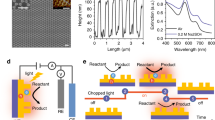
Abstract
Multi-electron redox reactions, although central to artificial photosynthesis, are kinetically sluggish. Amidst the search for synthetic catalysts for such processes, plasmonic nanoparticles have been found to catalyse multi-electron reduction of CO2 under visible light. This example motivates the need for a general, insight-driven framework for plasmonic catalysis of such multi-electron chemistry. Here, we elucidate the principles underlying the extraction of multiple redox equivalents from a plasmonic photocatalyst. We measure the kinetics of electron harvesting from a gold nanoparticle photocatalyst as a function of photon flux. Our measurements, supported by theoretical modelling, reveal a regime where two-electron transfer from the excited gold nanoparticle becomes prevalent. Multiple electron harvesting becomes possible under continuous-wave, visible-light excitation of moderate intensity due to strong interband transitions in gold and electron–hole separation accomplished using a hole scavenger. These insights will help expand the utility of plasmonic photocatalysis beyond CO2 reduction to other challenging multi-electron, multi-proton transformations such as N2 fixation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Linic, S., Christopher, P. & Ingram, D. B. Plasmonic-metal nanostructures for efficient conversion of solar to chemical energy. Nat. Mater. 10, 911–921 (2011).
Liu, Z., Hou, W., Pavaskar, P., Aykol, M. & Cronin, S. B. Plasmon resonant enhancement of photocatalytic water splitting under visible illumination. Nano Lett. 11, 1111–1116 (2011).
Yu, S., Wilson, A. J., Heo, J. & Jain, P. K. Plasmonic control of multi-electron transfer and C–C coupling in visible-light-driven CO2 reduction on Au nanoparticles. Nano Lett. 18, 2189–2194 (2018).
Yu, S., Wilson, A. J., Kumari, G., Zhang, X. & Jain, P. K. Opportunities and challenges of solar-energy-driven carbon dioxide to fuel conversion with plasmonic catalysts. ACS Energy Lett. 2, 2058–2070 (2017).
Christopher, P., Xin, H. & Linic, S. Visible-light-enhanced catalytic oxidation reactions on plasmonic silver nanostructures. Nat. Chem. 3, 467–472 (2011).
Christopher, P., Xin, H., Marimuthu, A. & Linic, S. Singular characteristics and unique chemical bond activation mechanisms of photocatalytic reactions on plasmonic nanostructures. Nat. Mater. 11, 1044–1050 (2012).
Marimuthu, A., Zhang, J. & Linic, S. Tuning selectivity in propylene. Science 339, 1590–1593 (2013).
Mukherjee, S. et al. Hot electrons do the impossible: plasmon-induced dissociation of H2 on Au. Nano Lett. 13, 240–247 (2013).
Mukherjee, S. et al. Hot-electron-induced dissociation of H2 on gold nanoparticles supported on SiO2. J. Am. Chem. Soc. 136, 64–67 (2013).
Mubeen, S. et al. An autonomous photosynthetic device in which all charge carriers derive from surface plasmons. Nat. Nanotechnol. 8, 247–251 (2013).
Hou, W. et al. Photocatalytic conversion of CO2 to hydrocarbon fuels via plasmon-enhanced absorption and metallic interband transitions. ACS Catal. 1, 929–936 (2011).
Clavero, C. Plasmon-induced hot-electron generation at nanoparticle/metal-oxide interfaces for photovoltaic and photocatalytic devices. Nat. Photonics 8, 95–103 (2014).
Watanabe, K., Menzel, D., Nilius, N. & Freund, H.-J. Photochemistry on metal nanoparticles. Chem. Rev. 106, 4301–4320 (2006).
Bigot, J., Halté, V., Merle, J. & Daunois, A. Electron dynamics in metallic nanoparticles. Chem. Phys. 25, 181–203 (2000).
Sheikholeslami, S., Jun, Y., Jain, P. K. & Alivisatos, A. P. Coupling of optical resonances in a compositionally asymmetric plasmonic nanoparticle dimer. Nano Lett. 10, 2655–2660 (2010).
Kim, K. H., Watanabe, K., Mulugeta, D., Freund, H.-J. & Menzel, D. Enhanced photoinduced desorption from metal nanoparticles by photoexcitation of confined hot electrons using femtosecond laser pulses. Phys. Rev. Lett. 107, 047401 (2011).
Nosaka, Y., Ohta, N. & Miyama, H. Photochemical kinetics of ultrasmall semiconductor particles in solution: effect of size on the quantum yield of electron transfer. J. Phys. Chem. 94, 3752–3755 (1990).
Mulugeta, D., Kim, K. H., Watanabe, K., Menzel, D. & Freund, H.-J. Size effects in thermal and photochemistry of (NO)2 on Ag nanoparticles. Phys. Rev. Lett. 101, 146103 (2008).
Mulugeta, D., Watanabe, K., Menzel, D. & Freund, H.-J. State-resolved investigation of the photodesorption dynamics of NO from (NO)2 on Ag nanoparticles of various sizes in comparison with Ag(111). J. Chem. Phys. 134, 164702 (2011).
Link, S. & El-sayed, M. A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 103, 8410–8426 (1999).
Liu, Z. et al. Understanding the growth mechanisms of Ag nanoparticles controlled by plasmon-induced charge transfers in Ag–TiO2 films. J. Phys. Chem. C 119, 9496–9505 (2015).
Redmond, P. L., Wu, X. & Brus, L. Photovoltage and photocatalyzed growth in citrate-stabilized colloidal silver nanocrystals. J. Phys. Chem. C. 111, 8942–8947 (2007).
Wu, X., Thrall, E. S., Liu, H., Steigerwald, M. & Brus, L. Plasmon induced photovoltage and charge separation in citrate-stabilized gold nanoparticles. J. Phys. Chem. C 114, 12896–12899 (2010).
Jain, P. K., Qian, W. & El-Sayed, M. A. Ultrafast cooling of photoexcited electrons in gold nanoparticle−thiolated DNA conjugates involves the dissociation of the gold−thiol bond. J. Am. Chem. Soc. 128, 2426–2433 (2006).
Smith, J. G. & Jain, P. K. The ligand shell as an energy barrier in surface reactions on transition metal nanoparticles. J. Am. Chem. Soc. 138, 6765–6773 (2016).
Alsan, M. et al. Polyvinylpyrrolidone as binder for castable supercapacitor electrodes with high electrochemical performance in organic electrolytes. J. Power Sources 266, 374–383 (2014).
Bauer, C., Abid, J.-P., Fermin, D. & Girault, H. H. Ultrafast chemical interface scattering as an additional decay channel for nascent nonthermal electrons in small metal nanoparticles. J. Chem. Phys. 120, 9302–9315 (2004).
Hövel, H., Fritz, S., Hilger, A., Kreibig, U. & Vollmer, M. Width of cluster plasmon resonances: bulk dielectric functions and chemical interface damping. Phys. Rev. B 48, 18178–18188 (1993).
Persson, B. N. J. Polarizability of small spherical metal particles: influence of the matrix environment. Surf. Sci. 281, 153–162 (1993).
Bard, A. J., Jordan, J. & Parsons, R. Standard Potentials in Aqueous Solutions (Marcel Dekker, New York, 1985).
Kolthoff, I. M. & Tomsicek, W. J. The oxidation potential of the system potassium ferrocyanide–potassium ferricyanide at various ionic strengths. J. Phys. Chem. 39, 945–954 (1934).
Carregal-Romero, S., Pérez-Juste, J., Hervés, P., Liz-Marzán, L. M. & Mulvaney, P. Colloidal gold-catalyzed reduction of ferrocyanate (III) by borohydride ions: a model system for redox catalysis. Langmuir 26, 1271–1277 (2010).
Fleischmann, M., Graves, P. R. & Robinson, J. The Raman spectroscopy of the ferricyanide/ferrocyanide system at gold, β-palladium hydride and platinum electrodes. J. Electroanal. Soc. 182, 87–98 (1985).
Siahrostami, S., Li, G.-L., Viswanathan, V. & Nørskov, J. K. One- or two-electron water oxidation, hydroxyl radical, or H2O2 evolution. J. Phys. Chem. Lett. 8, 1157–1160 (2017).
Jain, P. K., Lee, K. S., El-Sayed, I. H. & El-Sayed, M. A. Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine. J. Phys. Chem. B 110, 7238–7248 (2006).
Nosaka, Y., Ohta, N. & Miyama, H. Photochemical kinetics of ultrasmall semiconductor particles in solution: effect of size on the quantum yield of electron transfer. J. Phys. Chem. 94, 3752–3755 (1990).
Simon, T. et al. Redox shuttle mechanism enhances photocatalytic H2 generation on Ni-decorated CdS nanorods. Nat. Mater. 13, 1013–1018 (2014).
Dainton, F. S., Janovsky, I. V. & Salmon, G. A. Evidence for the production of an oxidising radical on pulse-radiolysis of methanol. J. Chem. Soc. D Chem. Comm. 0, 335–336 (1969).
Choi, J., Ryu, S. Y., Balcerski, W., Lee, T. K. & Hoffmann, M. R. Photocatalytic production of hydrogen on Ni/NiO/KNbO3/CdS nanocomposites using visible light. J. Mater. Chem. 18, 2371–2378 (2008).
Papaconstantinou, E. Relative electron-donating ability of simple alcohol radicals toward 12-heteropoly tungstates. J. Chem. Soc. Faraday Trans. 78, 2769–2772 (1982).
Papaconstantinou, E. New easy method for obtaining approximate redox potential of radicals, produced by 60Co-γ-radiolysis, using heteropoly electrolytes of molybdenum and tungsten as electron acceptors. The redox potential of some alcohol and organic acid radicals. Anal. Chem. 47, 1592–1595 (1975).
Maeda, K., Ozaki, N. & Akimoto, I. Alcohol additive effect in hydrogen generation from water with carbon by photochemical reaction. Jpn. J. Appl. Phys. 53, 1–3 (2014).
Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 94, 2319–2358 (1994).
Mooradian, A. Photoluminescence of metals. Phys. Rev. Lett. 22, 185–187 (1969).
Zoric, I., Zach, M., Kasemo, B. & Langhammer, C. Gold, platinum, and aluminum nanodisk plasmons: material damping mechanisms. ACS Nano 5, 2535–2546 (2011).
Sarina, S. et al. Viable photocatalysts under solar-spectrum irradiation: nonplasmonic metal nanoparticles. Angew. Chem. Int. Ed. Engl. 53, 2935–2940 (2014).
Zhao, J. et al. A comparison of photocatalytic activities of gold nanoparticles following plasmonic and interband excitation and a strategy for harnessing interband hot carriers for solution phase photocatalysis. ACS Cent. Sci. 3, 482–488 (2017).
Bird, R. E., Hulstrom, R. L. & Lewis, L. J. Terrestrial solar spectral data sets. Sol. Energy 30, 563–573 (1983).
Turkevich, J., Stevenson, P. C. & Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 11, 55–75 (1951).
Acknowledgements
We acknowledge funding through an Arnold and Mabel Beckman Foundation Young Investigator Award. J.G.S. was supported by a CAREER award to P.K.J. from the National Science Foundation (rant NSF CHE-1455011). The work was carried out in part at the Frederick Seitz Materials Research Laboratory at UIUC.
Author information
Authors and Affiliations
Contributions
P.K.J. conceived the project and designed the experiments. Y.K. conducted the experiments. P.K.J. performed the theoretical modelling. Y.K. and P.K.J analysed the data. J.G.S. performed the structural characterization. Y.K. and P.K.J. co-wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–13, Supplementary Tables 1 and 2, Supplementary Methods and Analysis
Rights and permissions
About this article
Cite this article
Kim, Y., Smith, J.G. & Jain, P.K. Harvesting multiple electron–hole pairs generated through plasmonic excitation of Au nanoparticles. Nature Chem 10, 763–769 (2018). https://doi.org/10.1038/s41557-018-0054-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0054-3
This article is cited by
-
Plasmonic bimetallic two-dimensional supercrystals for H2 generation
Nature Catalysis (2023)
-
Surface plasmon-enhanced photo-driven CO2 hydrogenation by hydroxy-terminated nickel nitride nanosheets
Nature Communications (2023)
-
Near-infrared-featured broadband CO2 reduction with water to hydrocarbons by surface plasmon
Nature Communications (2023)
-
Plasmon-mediated chemical reactions
Nature Reviews Methods Primers (2023)
-
Direct observation of the plasmon-enhanced palladium catalysis with single-molecule fluorescence microscopy
Nano Research (2023)