Abstract
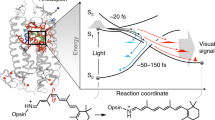
Vibronic coupling is key to efficient energy flow in molecular systems and a critical component of most mechanisms invoking quantum effects in biological processes. Despite increasing evidence for coherent coupling of electronic states being mediated by vibrational motion, it is not clear how and to what degree properties associated with vibrational coherence such as phase and coupling of atomic motion can impact the efficiency of light-induced processes under natural, incoherent illumination. Here, we show that deuteration of the H11–C11=C12–H12 double-bond of the 11-cis retinal chromophore in the visual pigment rhodopsin significantly and unexpectedly alters the photoisomerization yield while inducing smaller changes in the ultrafast isomerization dynamics assignable to known isotope effects. Combination of these results with non-adiabatic molecular dynamics simulations reveals a vibrational phase-dependent isotope effect that we suggest is an intrinsic attribute of vibronically coherent photochemical processes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Born, M. & Oppenheimer, R. Zur Quantentheorie der Molekeln. Ann. Phys. 389, 457–484 (1927).
Butler, L. J. Chemical reaction dynamics beyond the Born–Oppenheimer approximation. Annu. Rev. Phys. Chem. 49, 125–171 (1998).
Brixner, T. et al. Two-dimensional spectroscopy of electronic couplings in photosynthesis. Nature 434, 625–628 (2005).
Engel, G. S. et al. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446, 782–786 (2007).
Lee, H., Cheng, Y.-C. & Fleming, G. R. Coherence dynamics in photosynthesis: protein protection of excitonic coherence. Science 316, 1462–1465 (2007).
Romero, E. et al. Quantum coherence in photosynthesis for efficient solar-energy conversion. Nat. Phys. 10, 676–682 (2014).
Dostál, J., Pšenčík, J. & Zigmantas, D. In situ mapping of the energy flow through the entire photosynthetic apparatus. Nat. Chem. 8, 705–710 (2016).
Delor, M. et al. On the mechanism of vibrational control of light-induced charge transfer in donor–bridge–acceptor assemblies. Nat. Chem. 7, 689–695 (2015).
Lim, J. S., Lee, Y. S. & Kim, S. K. Control of intramolecular orbital alignment in the photodissociation of thiophenol: conformational manipulation by chemical substitution. Angew. Chem. Int. Ed. 47, 1853–1856 (2008).
Lim, J. S. & Kim, S. K. Experimental probing of conical intersection dynamics in the photodissociation of thioanisole. Nat. Chem. 2, 627–632 (2010).
Polli, D. et al. Conical intersection dynamics of the primary photoisomerization event in vision. Nature 467, 440–443 (2010).
Kukura, P., McCamant, D. W., Yoon, S., Wandschneider, D. B. & Mathies, R. A. Structural observation of the primary isomerization in vision with femtosecond-stimulated Raman. Science 310, 1006–1009 (2005).
Schapiro, I. et al. The ultrafast photoisomerizations of rhodopsin and bathorhodopsin are modulated by bond length alternation and HOOP driven electronic effects. J. Am. Chem. Soc. 133, 3354–3364 (2011).
Frutos, L. M., Andruniów, T., Santoro, F., Ferré, N. & Olivucci, M. Tracking the excited-state time evolution of the visual pigment with multiconfigurational quantum chemistry. Proc. Natl Acad. Sci. USA 104, 7764–7769 (2007).
Strambi, A., Coto, P. B., Frutos, L. M., Ferré, N. & Olivucci, M. Relationship between the excited state relaxation paths of rhodopsin and isorhodopsin. J. Am. Chem. Soc. 130, 3382–3388 (2008).
Schnedermann, C., Liebel, M. & Kukura, P. Mode-specificity of vibrationally coherent internal conversion in rhodopsin during the primary visual event. J. Am. Chem. Soc. 137, 2886–2891 (2015).
Wang, Q., Schoenlein, R. W., Peteanu, L. A., Mathies, R. A. & Shank, C. V. Vibrationally coherent photochemistry in the femtosecond primary event of vision. Science 266, 422–424 (1994).
Johnson, P. J. M. et al. Local vibrational coherences drive the primary photochemistry of vision. Nat. Chem. 7, 980–986 (2015).
Mathies, R. A. Photochemistry: a coherent picture of vision. Nat. Chem. 7, 945–947 (2015).
Garavelli, M., Celani, P., Bernardi, F., Robb, M. A. & Olivucci, M. The C5H6NH2 + protonated Shiff base: an ab initio minimal model for retinal photoisomerization. J. Am. Chem. Soc. 119, 6891–6901 (1997).
Sinicropi, A., Migani, A., De Vico, L. & Olivucci, M. Photoisomerization acceleration in retinal protonated Schiff-base models. Photochem. Photobiol. Sci. 2, 1250 (2003).
Garavelli, M. et al. Photoisomerization path for a realistic retinal chromophore model: the nonatetraeniminium cation. J. Am. Chem. Soc. 120, 1285–1288 (1998).
Gozem, S. et al. Mapping the excited state potential energy surface of a retinal chromophore model with multireference and equation-of-motion coupled-cluster methods. J. Chem. Theory Comput. 9, 4495–4506 (2013).
Weingart, O. & Garavelli, M. Modelling vibrational coherence in the primary rhodopsin photoproduct. J. Chem. Phys. 137, 22A523 (2012).
Weingart, O. et al. Product formation in rhodopsin by fast hydrogen motions. Phys. Chem. Chem. Phys. 13, 3645–3648 (2011).
Duan, H.-G., Miller, R. J. D. & Thorwart, M. Impact of vibrational coherence on the quantum yield at a conical intersection. J. Phys. Chem. Lett. 7, 3491–3496 (2016).
Qi, D.-L., Duan, H.-G., Sun, Z.-R., Miller, R. J. D. & Thorwart, M. Tracking an electronic wave packet in the vicinity of a conical intersection. J. Chem. Phys. 147, 74101 (2017).
Mathies, R. A. & Lugtenburg, J. Chapter 2. The primary photoreaction of rhodopsin. Handb. Biol. Phys. 3, 55–90 (2000).
Laptenok, S. P. et al. Complete proton transfer cycle in GFP and its T203V and S205V mutants. Angew. Chem. Int. Ed. 54, 9303–9307 (2015).
Klinman, J. P. & Kohen, A. Hydrogen tunneling links protein dynamics to enzyme catalysis. Annu. Rev. Biochem 82, 471–496 (2013).
Peters, K., Applebury, M. L. & Rentzepis, P. M. Primary photochemical event in vision: proton translocation. Proc. Natl Acad. Sci. USA 74, 3119–3123 (1977).
Fransen, M. R. et al. Structure of the chromophoric group in bathorhodopsin. Nature 260, 726–727 (1976).
Kim, J. E., Tauber, M. J. & Mathies, R. A. wavelength dependent cis–trans isomerization in vision. Biochemistry 40, 13774–13778 (2001).
Kochendoerfer, G. G., Verdegem, P. J., van der Hoef, I., Lugtenburg, J. & Mathies, R. A. Retinal analog study of the role of steric interactions in the excited state isomerization dynamics of rhodopsin. Biochemistry 35, 16230–16240 (1996).
Lin, S. W. et al. Vibrational assignment of torsional normal modes of rhodopsin: probing excited-state isomerization dynamics along the reactive C11=C12 torsion coordinate. J. Phys. Chem. B 102, 2787–2806 (1998).
Liebel, M., Schnedermann, C., Wende, T. & Kukura, P. Principles and applications of broadband impulsive vibrational spectroscopy. J. Phys. Chem. A 119, 9506–9517 (2015).
Bassolino, G. et al. Barrierless photoisomerization of 11-cis retinal protonated Schiff base in solution. J. Am. Chem. Soc. 137, 12434–12437 (2015).
Kovalenko, S. A., Dobryakov, A. L., Ruthmann, J. & Ernsting, N. P. Femtosecond spectroscopy of condensed phases with chirped supercontinuum probing. Phys. Rev. A 59, 2369–2384 (1999).
Schoenlein, R. W., Peteanu, L. A., Mathies, R. A. & Shank, C. V. The first step in vision: femtosecond isomerization of rhodopsin. Science 254, 412–415 (1991).
Dobryakov, A. L. et al. Femtosecond pump/supercontinuum-probe spectroscopy: optimized setup and signal analysis for single-shot spectral referencing. Rev. Sci. Instrum. 81, 113106 (2010).
Zener, C. Non-adiabatic crossing of energy levels. Proc. R. Soc. A 137, 696–702 (1932).
Landau, L. D. On the theory of transfer of energy at collisions II. Phys. Z. Sowjetunion 2, 7 (1932).
Ockenfels, A., Schapiro, I. & Gärtner, W. Rhodopsins carrying modified chromophores – the ‘making of’, structural modelling and their light-induced reactivity. Photochem. Photobiol. Sci. 15, 297–308 (2016).
Eyring, G., Curry, B., Broek, A., Lugtenburg, J. & Mathies, R. A. Assignment and interpretation of hydrogen out-of-plane vibrations in the resonance Raman spectra of rhodopsin and bathorhodopsin. Biochemistry 21, 384–393 (1982).
Gozem, S., Luk, H. L., Schapiro, I. & Olivucci, M. Theory and simulation of the ultrafast double-bond isomerization of biological chromophores. Chem. Rev. 117, 13502–13565 (2017).
Liebel, M., Schnedermann, C. & Kukura, P. Sub-10-fs pulses tunable from 480 to 980 nm from a NOPA pumped by an Yb:KGW source. Opt. Lett. 39, 4112–4115 (2014).
Sovdat, T. et al. Backbone modification of retinal induces protein-like excited state dynamics in solution. J. Am. Chem. Soc. 134, 8318–8320 (2012).
Okada, T. et al. The retinal conformation and its environment in rhodopsin in light of a new 2.2Å crystal structure. J. Mol. Biol. 342, 571–583 (2004).
Luk, H. L. et al. Modulation of thermal noise and spectral sensitivity in Lake Baikal cottoid fish rhodopsins. Sci. Rep. 6, 38425 (2016).
Cornell, W. D. et al. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 117, 5179–5197 (1995).
Manathunga, M. et al. Probing the photodynamics of rhodopsins with reduced retinal chromophores. J. Chem. Theory Comput. 12, 839–850 (2016).
Tully, J. C. Molecular dynamics with electronic transitions. J. Chem. Phys. 93, 1061–1071 (1990).
Granucci, G. & Persico, M. Critical appraisal of the fewest switches algorithm for surface hopping. J. Chem. Phys. 126, 134114 (2007).
Aquilante, F. et al. Molcas 8: new capabilities for multiconfigurational quantum chemical calculations across the periodic table. J. Comput. Chem. 37, 506–541 (2016).
Ponder, J. W. & Richards, F. M. Tinker molecular modeling package. J. Comput. Chem. 8, 1016–1024 (1987).
Acknowledgements
We acknowledge support from the National Eye Institute for providing 11-cis retinal used to make the 11,12-H2 regenerated rhodopsin sample. P.K. was supported by the EPSRC (EP/K006630/1). M.O. is supported by the NSF (CHE-1710191) and HFSP (RGP0049/2012) and also thanks the Ohio Supercomputer Center for computer time. I.S. is supported by the ERC Starting Grant ‘PhotoMutant’ (678169). This work was supported in part by the Mathies Royalty Fund.
Author information
Authors and Affiliations
Contributions
R.A.M. and J.L. conceived the project. P.K., C.S. and M.L. designed all experiments and analysed the data. J.L. and I.F. synthesized the isotopomers. M.O. and X.Y. carried out the molecular dynamics simulations and developed the proposed theoretical model. I.S. and A.V. wrote the isotope simulation code. K.M.S. prepared the rhodopsin samples for all measurements. C.S., P.K., M.O. and R.A.M. wrote the manuscript with contributions from all other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Data and Analysis, Supplementary Figures 1–17, Tables 1–3
Rights and permissions
About this article
Cite this article
Schnedermann, C., Yang, X., Liebel, M. et al. Evidence for a vibrational phase-dependent isotope effect on the photochemistry of vision. Nature Chem 10, 449–455 (2018). https://doi.org/10.1038/s41557-018-0014-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0014-y
This article is cited by
-
Ultrafast structural changes direct the first molecular events of vision
Nature (2023)
-
On the fluorescence enhancement of arch neuronal optogenetic reporters
Nature Communications (2022)
-
Direct structural observation of ultrafast photoisomerization dynamics in sinapate esters
Communications Chemistry (2022)
-
Evidence of exciton-libron coupling in chirally adsorbed single molecules
Nature Communications (2022)
-
Quantum–classical simulations of rhodopsin reveal excited-state population splitting and its effects on quantum efficiency
Nature Chemistry (2022)