Abstract
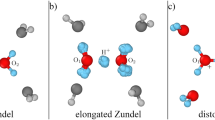
Proton transfer via hydronium and hydroxide ions in water is ubiquitous. It underlies acid–base chemistry, certain enzyme reactions, and even infection by the flu. Despite two centuries of investigation, the mechanism underlying why hydroxide diffuses slower than hydronium in water is still not well understood. Herein, we employ state-of-the-art density-functional-theory-based molecular dynamics—with corrections for non-local van der Waals interactions, and self-interaction in the electronic ground state—to model water and hydrated water ions. At this level of theory, we show that structural diffusion of hydronium preserves the previously recognized concerted behaviour. However, by contrast, proton transfer via hydroxide is less temporally correlated, due to a stabilized hypercoordination solvation structure that discourages proton transfer. Specifically, the latter exhibits non-planar geometry, which agrees with neutron-scattering results. Asymmetry in the temporal correlation of proton transfer leads to hydroxide diffusing slower than hydronium.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hückel, E. 3. Einzelvorträge: elektrochemie. theorie der beweglichkeiten des wasserstoff‐und hydroxylions in wässriger lösung. Z. Elektrochem. Angew. Phys. Chem. 34, 546–562 (1928).
Bernal, J. & Fowler, R. A theory of water and ionic solution, with particular reference to hydrogen and hydroxyl ions. J. Phys. Chem. 1, 515–548 (1933).
de Grotthuss, C. Theory of decomposition of liquids by electrical currents. [in French] Ann. Chim. 58, 54–74 (1806).
Agmon, N. et al. Protons and hydroxide ions in aqueous systems. Chem. Rev. 116, 7642–7672 (2016).
Halle, B. & Karlström, G. Prototropic charge migration in water. Part 2. Interpretation of nuclear magnetic resonance and conductivity data in terms of model mechanisms. J. Chem. Soc. Faraday Trans. 2 79, 1047–1073 (1983).
Weingärtner, H. & Chatzidimttriou-Dreismann, C. Anomalous H+ and D+ conductance in H2O–D2O mixtures. Nature 346, 548–550 (1990).
Sluyters, J. & Sluyters-Rehbach, M. The mechanism of the hydrogen ion conduction in liquid light and heavy water derived from the temperature dependence of their limiting conductivities. J. Phys. Chem. B 114, 15582–15589 (2010).
Halle, B. & Karlström, G. Prototropic charge migration in water. Part 1. Rate constants in light and heavy water and in salt solution from oxygen-17 spin relaxation. J. Chem. Soc. Faraday Trans. 2 79, 1031–1046 (1983).
Car, R. & Parrinello, M. Unified approach for molecular dynamics and density-functional theory. Phys. Rev. Lett. 55, 2471 (1985).
Hohenberg, P. & Kohn, W. Inhomogeneous electron gas. Phys. Rev. 136, B864–B871 (1964).
Kohn, W. & Sham, L. J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 140, A1133–A1138 (1965).
Tuckerman, M. E., Laasonen, K., Sprik, M. & Parrinello, M. Ab initio simulations of water and water ions. J. Phys. Condens. Matter 6, A93 (1994).
Wicke, E., Eigen, M. & Ackermann, T. Über den zustand des protons (hydroniumions) in wäβriger lösung. Z. Phys. Chem. 1, 340 (1954).
Eigen, M. Proton transfer, acid–base catalysis, and enzymatic hydrolysis. Part I: Elementary processes. Angew. Chem. Int. Ed. 3, 1–19 (1964).
Zundel, G. & Metzger, H. Energiebänder der tunnelnden überschuß-protonen in flüssigen säuren. Eine IR-spektroskopische untersuchung der natur der gruppierungen H5O2 +. Z. Phys. Chem. 58, 225–245 (1968).
Tse, Y.-L. S., Knight, C. & Voth, G. A. An analysis of hydrated proton diffusion in ab initio molecular dynamics. J. Chem. Phys. 142, 014104 (2015).
Thämer, M., De Marco, L., Ramasesha, K., Mandal, A. & Tokmakoff, A. Ultrafast 2D IR spectroscopy of the excess proton in liquid water. Science 350, 78–82 (2015).
Decka, D., Schwaab, G. & Havenith, M. A. THz/FTIR fingerprint of the solvated proton: evidence for Eigen structure and Zundel dynamics. Phys. Chem. Chem. Phys. 17, 11898–11907 (2015).
Dahms, F. et al. The hydrated excess proton in the Zundel cation H5O2 +: the role of ultrafast solvent fluctuations. Angew. Chem. Int. Ed. 55, 10600–10605 (2016).
Wolke, C. T. et al. Spectroscopic snapshots of the proton-transfer mechanism in water. Science 354, 1131–1135 (2016).
Dahms, F., Fingerhut, B. P., Nibbering, E. T., Pines, E. & Elsaesser, T. Large-amplitude transfer motion of hydrated excess protons mapped by ultrafast 2D IR spectroscopy. Science 357, 491–495 (2017).
Biswas, R., Carpenter, W., Fournier, J. A., Voth, G. A. & Tokmakoff, A. IR spectral assignments for the hydrated excess proton in liquid water. J. Phys. Chem. 146, 154507 (2017).
Daly, C. A. et al. Decomposition of the experimental Raman and infrared spectra of acidic water into proton, special pair, and counter-ion contributions. J. Phys. Chem. Lett. 8, 5246–5252 (2017).
Botti, A., Bruni, F., Imberti, S., Ricci, M. & Soper, A. Solvation of hydroxyl ions in water. J. Phys. Chem. 119, 5001–5004 (2003).
Aziz, E. F., Ottosson, N., Faubel, M., Hertel, I. V. & Winter, B. Interaction between liquid water and hydroxide revealed by core–hole de-excitation. Nature 455, 89–91 (2008).
Tuckerman, M. E., Chandra, A. & Marx, D. Structure and dynamics of OH–(aq). Acc. Chem. Res. 39, 151–158 (2006).
Marx, D. Proton transfer 200 years after von Grotthuss: insights from ab initio simulations. ChemPhysChem 7, 1848–1870 (2006).
Hassanali, A., Prakash, M. K., Eshet, H. & Parrinello, M. On the recombination of hydronium and hydroxide ions in water. Proc. Natl Acad. Sci. USA 108, 20410–20415 (2011).
Hassanali, A., Giberti, F., Cuny, J., Kühne, T. D. & Parrinello, M. Proton transfer through the water gossamer. Proc. Natl Acad. Sci. USA 110, 13723–13728 (2013).
Gillan, M. J., Alfè, D. & Michaelides, A. Perspective: how good is DFT for water? J. Phys. Chem. 144, 130901 (2016).
Gaiduk, A. P., Gygi, F. & Galli, G. Density and compressibility of liquid water and ice from first-principles simulations with hybrid functionals. J. Phys. Chem. Lett. 6, 2902–2908 (2015).
Miceli, G., de Gironcoli, S. & Pasquarello, A. Isobaric first-principles molecular dynamics of liquid water with nonlocal van der Waals interactions. J. Chem. Phys. 142, 034501 (2015).
Marx, D., Chandra, A. & Tuckerman, M. E. Aqueous basic solutions: hydroxide solvation, structural diffusion, and comparison to the hydrated proton. Chem. Rev. 110, 2174–2216 (2010).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Phys. Chem. 105, 9982–9985 (1996).
Wu, X. F., Selloni, A. & Car, R. Order-N implementation of exact exchange in extended insulating systems. Phys. Rev. B 79, 085102 (2009).
Tkatchenko, A. & Scheffler, M. Accurate molecular van der Waals interactions from ground-state electron density and free-atom reference data. Phys. Rev. Lett. 102, 073005 (2009).
DiStasio, R. A. Jr, Santra, B., Li, Z., Wu, X. & Car, R. The individual and collective effects of exact exchange and dispersion interactions on the ab initio structure of liquid water. J. Phys. Chem. 141, 084502 (2014).
Crespo, Y. & Hassanali, A. Unveiling the Janus-like properties of OH–. J. Phys. Chem. Lett. 6, 272–278 (2015).
Marzari, N. & Vanderbilt, D. Maximally localized generalized Wannier functions for composite energy bands. Phys. Rev. B 56, 12847–12865 (1997).
Marzari, N., Mostofi, A. A., Yates, J. R., Souza, I. & Vanderbilt, D. Maximally localized Wannier functions: theory and applications. Rev. Mod. Phys. 84, 1419 (2012).
Hassanali, A. A., Giberti, F., Sosso, G. C. & Parrinello, M. The role of the umbrella inversion mode in proton diffusion. Chem. Phys. Lett. 599, 133–138 (2014).
Wang, F., Izvekov, S. & Voth, G. A. Unusual “amphiphilic” association of hydrated protons in strong acid solution. J. Am. Chem. Soc. 130, 3120–3126 (2008).
Iuchi, S., Chen, H., Paesani, F. & Voth, G. A. Hydrated excess proton at water−hydrophobic interfaces. J. Phys. Chem. B 113, 4017–4030 (2008).
Tse, Y. L., Chen, C., Lindberg, G. E., Kumar, R. & Voth, G. A. Propensity of hydrated excess protons and hydroxide anions for the air–water interface. J. Am. Chem. Soc. 137, 12610 (2015).
Giberti, F. & Hassanali, A. The excess proton at the air–water interface: the role of instantaneous liquid interfaces. J. Chem. Phys. 146, 244703 (2017).
Woutersen, S. & Bakker, H. J. Ultrafast vibrational and structural dynamics of the proton in liquid water. Phys. Rev. Lett. 96, 138305 (2006).
Tielrooij, K. J., Timmer, R. L. A., Bakker, H. J. & Bonn, M. Structure dynamics of the proton in liquid water probed with terahertz time-domain spectroscopy. Phys. Rev. Lett. 102, 198303 (2009).
Marx, D., Tuckerman, M. E., Hutter, J. & Parrinello, M. The nature of the hydrated excess proton in water. Nature 397, 601–604 (1999).
Chen, J., Li, X.-Z., Zhang, Q., Michaelides, A. & Wang, E. Nature of proton transport in a water-filled carbon nanotube and in liquid water. Phys. Chem. Chem. Phys. 15, 6344–6349 (2013).
Giberti, F., Hassanali, A. A., Ceriotti, M. & Parrinello, M. The role of quantum effects on structural and electronic fluctuations in neat and charged water. J. Phys. Chem. B 118, 13226–13235 (2014).
Giannozzi, P. et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 29, 465901 (2017).
Troullier, N. & Martins, J. L. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B 43, 1993–2006 (1991).
Tassone, F., Mauri, F. & Car, R. Acceleration schemes for ab initio molecular-dynamics simulations and electronic-structure calculations. Phys. Rev. B 50, 10561 (1994).
Martyna, G. J., Klein, M. L. & Tuckerman, M. E. Nosé–Hoover chains: the canonical ensemble via continuous dynamics. J. Phys. Chem. 97, 2635–2643 (1992).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865 (1996).
Luzar, A. & Chandler, D. Hydrogen-bond kinetics in liquid water. Nature 379, 55–57 (1996).
Wang, Y. & Perdew, J. P. Correlation hole of the spin-polarized electron gas, with exact small-wave-vector and high-density scaling. Phys. Rev. B 44, 13298 (1991).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098 (1988).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation–energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988).
Boese, A. D., Doltsinis, N. L., Handy, N. C. & Sprik, M. New generalized gradient approximation functionals. J. Phys. Chem. 112, 1670–1678 (2000).
Acknowledgements
This project was supported by US Department of Energy SciDAC under grant numbers DE-SC0008726 and DE-SC0008626 and partially supported by the Division of Materials Research (DMR) under Award DMR-1552287. R.A.D. acknowledges partial support from Cornell University through start-up funding and the Cornell Center for Materials Research (CCMR) with funding from the National Science Foundation (NSF) MRSEC programme (DMR-1719875). This research used resources of the Argonne Leadership Computing Facility at Argonne National Laboratory, which is supported by the Office of Science of the US Department of Energy under contract number DE-AC02-06CH11357. This research also used resources of the National Energy Research Scientific Computing Center, which is supported by the Office of Science of the US Department of Energy under contract number DE-AC02-05CH11231. X.W. is grateful for the useful discussions with D. Vanderbilt at Rutgers University and A. J. Shanahan at University Medical Center of Princeton.
Author information
Authors and Affiliations
Contributions
X.W., R.C. and M.L.K. designed the project. M.C. and L.Z. carried out the simulations. M.C. and L.Z. performed the analysis. R.A.D., B.S. and H.-Y.K. developed methodologies in Quantum ESPRESSO. X.W., R.C., M.L.K. and R.A.D. wrote the manuscript. All authors contributed to the discussions and revisions of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2; Supplementary Tables 1 and 2; Supplementary Methods and Discussion
Rights and permissions
About this article
Cite this article
Chen, M., Zheng, L., Santra, B. et al. Hydroxide diffuses slower than hydronium in water because its solvated structure inhibits correlated proton transfer. Nature Chem 10, 413–419 (2018). https://doi.org/10.1038/s41557-018-0010-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-018-0010-2
This article is cited by
-
Mechanistic insight into the competition between interfacial and bulk reactions in microdroplets through N2O5 ammonolysis and hydrolysis
Nature Communications (2024)
-
Understanding hydrogen electrocatalysis by probing the hydrogen-bond network of water at the electrified Pt–solution interface
Nature Energy (2023)
-
Enhanced interfacial water dissociation on a hydrated iron porphyrin single-atom catalyst in graphene
Communications Chemistry (2023)
-
Self-consistent determination of long-range electrostatics in neural network potentials
Nature Communications (2022)
-
Investigating water/oil interfaces with opto-thermophoresis
Nature Communications (2022)