Abstract
Anchored cells of the basal epidermis constantly undergo proliferation in an overcrowded environment. An important regulator of epidermal proliferation is YAP, which can be controlled by both cell–matrix and cell–cell interactions. Here, we report that THY1, a GPI-anchored protein, inhibits epidermal YAP activity through converging molecular mechanisms. THY1 deficiency leads to increased adhesion by activating the integrin-β1–SRC module. Notably, regardless of high cellular densities, the absence of THY1 leads to the dissociation of an adherens junction complex that enables the release and translocation of YAP. Due to increased YAP-dependent proliferation, Thy1–/– mice display enhanced wound repair and hair follicle regeneration. Taken together, our work reveals THY1 as a crucial regulator of cell–matrix and cell–cell interactions that controls YAP activity in skin homeostasis and regeneration.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Further data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Fuchs, E. Skin stem cells: rising to the surface. J. Cell Biol. 180, 273–284 (2008).
Fuchs, E. Epithelial skin biology: three decades of developmental biology, a hundred questions answered and a thousand new ones to address. Curr. Top. Dev. Biol. 116, 357–374 (2016).
Watt, F. M. Mammalian skin cell biology: at the interface between laboratory and clinic. Science 346, 937–940 (2014).
Fuchs, E. & Raghavan, S. Getting under the skin of epidermal morphogenesis. Nat. Rev. Genet. 3, 199–209 (2002).
Sumigray, K. D. & Lechler, T. Cell adhesion in epidermal development and barrier formation. Curr. Top. Dev. Biol. 112, 383–414 (2015).
Watt, F. M. & Huck, W. T. Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 14, 467–473 (2013).
Camargo, F. D. et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr. Biol. 17, 2054–2060 (2007).
Moya, I. M. & Halder, G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 20, 211–226 (2019).
Piccolo, S., Dupont, S. & Cordenonsi, M. The biology of YAP/TAZ: hippo signaling and beyond. Physiol. Rev. 94, 1287–1312 (2014).
Schlegelmilch, K. et al. Yap1 acts downstream of α-catenin to control epidermal proliferation. Cell 144, 782–795 (2011).
Silvis, M. R. et al. α-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci. Signal. 4, ra33 (2011).
Yap, A. S., Duszyc, K. & Viasnoff, V. Mechanosensing and mechanotransduction at cell–cell junctions. old. Spring Harb. Perspect. Biol. 10, a028761 (2018).
Yosefzon, Y. et al. Caspase-3 regulates YAP-dependent cell proliferation and organ size. Mol. Cell 70, 573–587.e4 (2018).
Zhang, H., Pasolli, H. A. & Fuchs, E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc. Natl. Acad. Sci. USA 108, 2270–2275 (2011).
Li, P. et al. αE-catenin inhibits a Src–YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev. 30, 798–811 (2016).
Fu, V., Plouffe, S. W. & Guan, K. L. The Hippo pathway in organ development, homeostasis, and regeneration. Curr. Opin. Cell Biol. 49, 99–107 (2017).
Plouffe, S. W. et al. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. J. Biol. Chem. 293, 11230–11240 (2018).
Yu, F. X., Zhao, B. & Guan, K. L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 (2015).
Dupont, S. et al. Role of YAP/TAZ in mechanotransduction. Nature 474, 179–183 (2011).
Elbediwy, A. et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development 143, 1674–1687 (2016).
Mason, D. E. et al. YAP and TAZ limit cytoskeletal and focal adhesion maturation to enable persistent cell motility. J. Cell Biol. 218, 1369–1389 (2019).
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10, 207–217 (2009).
Hagood, J. S. Thy-1 as an integrator of diverse extracellular signals. Front. Cell Dev. Biol. 7, 26 (2019).
Hu, P. & Barker, T. H. Thy-1 in integrin mediated mechanotransduction. Front. Cell Dev. Biol. 7, 22 (2019).
Leyton, L. et al. Thy-1/CD90 a bidirectional and lateral signaling scaffold. Front. Cell Dev. Biol. 7, 132 (2019).
Nosten-Bertrand, M. et al. Normal spatial learning despite regional inhibition of LTP in mice lacking Thy-1. Nature 379, 826–829 (1996).
Johnson, R. & Halder, G. The two faces of Hippo: targeting the Hippo pathway for regenerative medicine and cancer treatment. Nat. Rev. Drug Discov. 13, 63–79 (2014).
Cordenonsi, M. et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147, 759–772 (2011).
Panciera, T., Azzolin, L., Cordenonsi, M. & Piccolo, S. Mechanobiology of YAP and TAZ in physiology and disease. Nat. Rev. Mol. Cell Biol. 18, 758–770 (2017).
Totaro, A., Panciera, T. & Piccolo, S. YAP/TAZ upstream signals and downstream responses. Nat. Cell Biol. 20, 888–899 (2018).
Beronja, S. & Fuchs, E. RNAi-mediated gene function analysis in skin. Methods Mol. Biol. 961, 351–361 (2013).
Beronja, S., Livshits, G., Williams, S. & Fuchs, E. Rapid functional dissection of genetic networks via tissue-specific transduction and RNAi in mouse embryos. Nat. Med. 16, 821–827 (2010).
Elbediwy, A., Vincent-Mistiaen, Z. I. & Thompson, B. J. YAP and TAZ in epithelial stem cells: a sensor for cell polarity, mechanical forces and tissue damage. Bioessays 38, 644–653 (2016).
Elosegui-Artola, A. et al. Force triggers YAP nuclear entry by regulating transport across nuclear pores. Cell 171, 1397–1410.e14 (2017).
Nardone, G. et al. YAP regulates cell mechanics by controlling focal adhesion assembly. Nat. Commun. 8, 15321 (2017).
Kashef, J. & Franz, C. M. Quantitative methods for analyzing cell–cell adhesion in development. Dev. Biol. 401, 165–174 (2015).
Fiore, V. F., Ju, L., Chen, Y., Zhu, C. & Barker, T. H. Dynamic catch of a Thy-1–α5β1+syndecan-4 trimolecular complex. Nat. Commun. 5, 4886 (2014).
De Luca, M., Pellegrini, G., Zambruno, G. & Marchisio, P. C. Role of integrins in cell adhesion and polarity in normal keratinocytes and human skin pathologies. J. Dermatol. 21, 821–828 (1994).
Brakebusch, C. et al. Skin and hair follicle integrity is crucially dependent on β1 integrin expression on keratinocytes. EMBO J. 19, 3990–4003 (2000).
Fish, K. N. Total internal reflection fluorescence (TIRF) microscopy. Curr. Protoc. Cytom. 0.12, Unit12.18 (2009).
Sabra, H. et al. β1 integrin-dependent Rac/group I PAK signaling mediates YAP activation of Yes-associated protein 1 (YAP1) via NF2/merlin. J. Biol. Chem. 292, 19179–19197 (2017).
Si, Y. et al. Src inhibits the Hippo tumor suppressor pathway through tyrosine phosphorylation of Lats1. Cancer Res. 77, 4868–4880 (2017).
Kim, N. G. & Gumbiner, B. M. Adhesion to fibronectin regulates Hippo signaling via the FAK–Src–PI3K pathway. J. Cell Biol. 210, 503–515 (2015).
Lamar, J. M. et al. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J. Biol. Chem. 294, 2302–2317 (2019).
Yu, F. X. et al. Regulation of the Hippo–YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012).
Wada, K., Itoga, K., Okano, T., Yonemura, S. & Sasaki, H. Hippo pathway regulation by cell morphology and stress fibers. Development 138, 3907–3914 (2011).
Cai, J. et al. A RhoA–YAP–c-Myc signaling axis promotes the development of polycystic kidney disease. Genes Dev. 32, 781–793 (2018).
Larjava, H. et al. Novel function for β1 integrins in keratinocyte cell–cell interactions. J. Cell Biol. 110, 803–815 (1990).
Chattopadhyay, N., Wang, Z., Ashman, L. K., Brady-Kalnay, S. M. & Kreidberg, J. A. α3β1 integrin–CD151, a component of the cadherin–catenin complex, regulates PTPμ expression and cell–cell adhesion. J. Cell Biol. 163, 1351–1362 (2003).
Kim, Y. et al. Integrin α3β1-dependent β-catenin phosphorylation links epithelial Smad signaling to cell contacts. J. Cell Biol. 184, 309–322 (2009).
Kim, N. G., Koh, E., Chen, X. & Gumbiner, B. M. E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc. Natl. Acad. Sci. USA 108, 11930–11935 (2011).
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Rognoni, E. et al. Inhibition of β-catenin signalling in dermal fibroblasts enhances hair follicle regeneration during wound healing. Development 143, 2522–2535 (2016).
Lee, M. J., Byun, M. R., Furutani-Seiki, M., Hong, J. H. & Jung, H. S. YAP and TAZ regulate skin wound healing. J. Invest. Dermatol. 134, 518–525 (2014).
Rognoni, E. & Walko, G. The roles of YAP/TAZ and the Hippo pathway in healthy and diseased skin. Cells 8, 411 (2019).
Mascharak, S. et al. Multi-omic analysis reveals divergent molecular events in scarring and regenerative wound healing. Cell Stem Cell 29, 315–327.e6 (2022).
Mascharak, S. et al. Preventing Engrailed-1 activation in fibroblasts yields wound regeneration without scarring. Science 372, eaba2374 (2021).
Ito, M. et al. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 (2007).
Ankawa, R. & Fuchs, Y. May the best wound WIHN: the hallmarks of wound-induced hair neogenesis. Curr. Opin. Genet. Dev. 72, 53–60 (2021).
Park, S. et al. Tissue-scale coordination of cellular behaviour promotes epidermal wound repair in live mice. Nat. Cell Biol. 19, 155–163 (2017).
Aragona, M. et al. Defining stem cell dynamics and migration during wound healing in mouse skin epidermis. Nat. Commun. 8, 14684 (2017).
Gay, D. et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 19, 916–923 (2013).
Lim, C. H. et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat. Commun. 9, 4903 (2018).
Schmidt, M. et al. Controlling the balance of fibroblast proliferation and differentiation: impact of Thy-1. J. Invest. Dermatol. 135, 1893–1902 (2015).
Driskell, R. R. et al. Distinct fibroblast lineages determine dermal architecture in skin development and repair. Nature 504, 277–281 (2013).
Liu-Chittenden, Y. et al. Genetic and pharmacological disruption of the TEAD–YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 26, 1300–1305 (2012).
Woo, W. M., Atwood, S. X., Zhen, H. H. & Oro, A. E. Rapid genetic analysis of epithelial-mesenchymal signaling during hair regeneration. J. Vis. Exp. https://doi.org/10.3791/4344 (2013).
El Ghalbzouri, A., Jonkman, M. F., Dijkman, R. & Ponec, M. Basement membrane reconstruction in human skin equivalents is regulated by fibroblasts and/or exogenously activated keratinocytes. J. Invest. Dermatol. 124, 79–86 (2005).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Romagnoli, M. et al. Deciphering the mammary stem cell niche: a role for laminin-binding integrins. Stem Cell Rep. 12, 831–844 (2019).
Zhou, Z. et al. α6-Integrin alternative splicing: distinct cytoplasmic variants in stem cell fate specification and niche interaction. Stem Cell Res. Ther. 9, 122 (2018).
Kobielak, A. & Fuchs, E. Links between α-catenin, NF-κB, and squamous cell carcinoma in skin. Proc. Natl. Acad. Sci. USA 103, 2322–2327 (2006).
Vasioukhin, V., Bauer, C., Degenstein, L., Wise, B. & Fuchs, E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of α-catenin in skin. Cell 104, 605–617 (2001).
Martin, G. S. The road to Src. Oncogene 23, 7910–7917 (2004).
Alzahrani, F. et al. The Hippo component YAP localizes in the nucleus of human papilloma virus positive oropharyngeal squamous cell carcinoma. J. Otolaryngol. Head Neck Surg. 46, 15 (2017).
Debaugnies, M. et al. YAP and TAZ are essential for basal and squamous cell carcinoma initiation. EMBO Rep. 19, e45809 (2018).
Vincent-Mistiaen, Z. YAP drives cutaneous squamous cell carcinoma formation and progression. eLife 7, e33304 (2018).
Wang, L. et al. Unbalanced YAP–SOX9 circuit drives stemness and malignant progression in esophageal squamous cell carcinoma. Oncogene 38, 2042–2055 (2019).
Avril, T. et al. CD90 expression controls migration and predicts dasatinib response in glioblastoma. Clin. Cancer Res. 23, 7360–7374 (2017).
He, J. et al. CD90 is identified as a candidate marker for cancer stem cells in primary high-grade gliomas using tissue microarrays. Mol. Cell Proteom. 11, M111 010744 (2012).
Lung, H. L. et al. THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene 24, 6525–6532 (2005).
Lu, H. et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 16, 1105–1117 (2014).
Sauzay, C., Voutetakis, K., Chatziioannou, A., Chevet, E. & Avril, T. CD90/Thy-1, a cancer-associated cell surface signaling molecule. Front. Cell Dev. Biol. 7, 66 (2019).
Tang, K. H. et al. A CD90+ tumor-initiating cell population with an aggressive signature and metastatic capacity in esophageal cancer. Cancer Res. 73, 2322–2332 (2013).
True, L. D. et al. CD90/THY1 is overexpressed in prostate cancer-associated fibroblasts and could serve as a cancer biomarker. Mod. Pathol. 23, 1346–1356 (2010).
Zhu, J., Thakolwiboon, S., Liu, X., Zhang, M. & Lubman, D. M. Overexpression of CD90 (Thy-1) in pancreatic adenocarcinoma present in the tumor microenvironment. PLoS ONE 9, e115507 (2014).
Soteriou, D. et al. Isolating hair follicle stem cells and epidermal keratinocytes from dorsal mouse skin. J. Vis. Exp. https://doi.org/10.3791/53931 (2016).
Kostic, L., Sedov, E., Soteriou, D., Yosefzon, Y. & Fuchs, Y. Isolation of stem cells and progenitors from mouse epidermis. Curr. Protoc. Stem Cell Biol. 41, 1C.20.1–1C.20.11 (2017).
Acknowledgements
We apologize to colleagues in the field whose important works we could not cite owing to space constraints. We thank all members of the Fuchs Lab as well as V. Sedov and O. Sedova for helpful comments; V. Zlobin at the PCRA for providing animal care; E. Barak at the Technion Flow Cytometry Unit for assisting with FACS experiments; and M. Allabi and K. Nafe for their warm support. Y.F. is the Deloro Chair and is funded by ICRF (2028184) and ISF (2027619) grants. Images were generated using BioRender software.
Author information
Authors and Affiliations
Contributions
E.S., E.K. and Y.F. designed and analysed the experiments. E.S., E.K. and S.C. performed the experiments. Y.Y. provided technical assistance throughout the project. L.E.W. and Y.S. performed TIRF imaging. R.A., A.M., A.S. and C.L. provided assistance and performed in utero microinjections. M.Y. and A.F. assisted in lentiviral construct designs. Y.F. conceived and supervised the project. E.S., E.K. and Y.F. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Cell Biology and the authors thank Valerie Horsley, Barry Thompson, and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Thy1-/- mice display increased numbers of hair follicles, but no difference in hair follicle cycling.
a,b, Quantifications for number (a) and distance (b) between adult (P56) dorsal hair follicles (HFs) (n = 5 mice). c, Immunofluorescence for KRT15 on tail skin wholemounts from adult (P56) wild type (WT) and Thy1-/- mice. d,e, Quantifications for average number (d) and distance (e) of tail skin HFs (n = 5 mice). f, Schematic of HF cycling after waxing of adult telogenic (P56) mice. Ana, anagen. g-i, Photographs of depilated WT and Thy1-/- mice at day 1 (g), day 5 (h), and day 9 (i) post waxing (n = 3 mice). j, Immunofluorescence for KRT5 on dorsal skin sections from WT and Thy1-/- mice at day 9 post waxing when mice are in late anagen (Ana-VI). k, Brightfield images of WT and Thy1-/- anagenic HFs. Dashed lines demarcate HFs. l, HF length at day 9 post waxing (n = 7 mice). All images and quantifications are representative of at least n = 3 mice per genotype, unless otherwise indicated. Quantifications were performed on fields of ≥ 60 samples ranging from 100-200μm, unless otherwise specified. Error bars indicate mean ± s.e.m. Scale bars, 50 μm (k), 100 μm (j) or 200 μm (c).
Extended Data Fig. 2 Loss of THY1 leads to enhanced YAP activation.
a, Representative confocal images of dorsal skin sections from wild type (WT; shown in inset) and Thy1-/- mice stained for pan-YAP and ITGβ4 (CD104). White arrows indicate nuclear YAP. b, YAPactive signal was normalized using β-actin as a loading control (relating to Fig. 2c) (n = 3 independent blots). c., Signal of pYAPS127 level normalized to α-tubulin (relating to Fig. 2e) (n = 3 independent blots). d, Immunostaining against active YAP in scrambled (shSCR) control mice and uninfected littermates (of shTHY1). e. Number of active YAP+ epidermal cells (n = 3 independent cultures). f, Scramble control (Ctrl) and THY1-silenced (shTHY1) keratinocytes (HaCaT) positive for GFP, demonstrating high lentiviral transduction efficiency (n ≥ 3 independent cultures). g, qPCR analysis of THY1 mRNA post infection (n = 3 independent cultures, analyzed in triplicate). Values were normalized to RPLP0. h, Whole lysate proteins isolated from Ctrl and shTHY1 HaCaT cells immunoblotted against THY1 and β-actin. i, Number of keratinocytes per colony in Ctrl and shTHY1 cultures (n = 5 independent cultures of each treatmen)]. j, Ctrl and shTHY1 keratinocytes immunostained against MCM2. k, Relative number of MCM2+ cells per colony (n = 15 colonies, from three independent cultures). l, Relative number of active YAP+ cells per colony (n = 15 colonies in three independent cultures). m, Whole lysate proteins isolated from Ctrl and shTHY1 non-confluent keratinocytes immunoblotted against YAPactive and β-actin. n, RNA isolated from Ctrl and THY1-OE (THY1-overexpression) subjected to qPCR analysis of THY1 (n = 3 independent cultures analyzed in triplicates). Values were normalized to RPLP0. o, Whole lysate proteins isolated from Ctrl and THY1-OE keratinocytes immunoblotted against THY1 and GAPDH. p, Densitometry of pYAPS127 signal normalized to β-actin protein levels. q, Percentage nuclear active YAP+ cells per field in confluent cultures (n = 10 colonies in three independent cultures). All images are representative of at least n = 3 mice of each genotype, unless otherwise stated. Error bars indicate the mean ± s.e.m. Scale bars: 20 μm (d,j) or 50 μm (a,f).
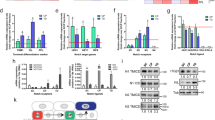
Extended Data Fig. 3 Lack of THY1 results in increased adhesion.
a, Quantification of relative cell size of Ctrl and shTHY1 keratinocytes (n = three independent cultures). b, Equal numbers of Ctrl and shTHY1 keratinocytes passaged and seeded, then monitored for adhesion (n = three independent cultures). c, Quantifications of cell adhesion of Ctrl and shTHY1 keratinocytes after seeding. d, Confluent Ctrl and shTHY1 keratinocytes were incubated with Trypsin/EDTA (0.25%) and monitored for detachment. e, Confluent ITGα6+/SCAI+ keratinocytes from WT and Thy1-/- mice were incubated with Trypsin/EDTA (0.25%) and monitored for detachment. f, Representative image of remaining (adhered) shSCR (Ctrl) and shTHY1 cells. g, Quantification of remaining (adhered) shSCR (Ctrl) and shTHY1 cells (n = four independent cultures). h, TIRF microcopy image of THY1-OE (THY1-overexpressed) keratinocytes immunostained for ITGβ1 and THY1. i, Ctrl, shTHY1 and THY-OE HaCaT cells immunoblotted against ITGβ1 and β-actin (n = 3 independent cultures of each). j, Dorsal skin cells immunoblotted against ITGβ1 and β-actin (n = 3 pooled mice each). k, Signal of pSRCY418 level normalized to β-actin (relating to Fig. 3k). l, Whole lysate proteins isolated from non-confluent and confluent Ctrl and shTHY1 HaCaT cells immunoblotted against pYAPY357 and β-actin. m, Immunostaining of active pYAPY357 in shSCR (control) and shTHY1 HaCaT cells. n, Primary dorsal keratinocytes treated with SRC inhibitor dasatinib immunostained for active ITGβ1 and active YAP. o, Relative percentage of active YAP+ primary keratinocytes. p, Whole lysate proteins isolated from Ctrl and shTHY1 HaCaT cells, treated with ROCK inhibitor (Y27632) and dasatinib, immunoblotted against active YAP and β-actin. All images are representative of at least n = 3 independent cultures per genotype, unless otherwise indicated. Scale bars, 5 μm (h), 20 μm (m) or 50 μm (f,n).
Extended Data Fig. 4 Co-localization of cell-cell and cell-matrix proteins.
a, Schematic of Förster resonance energy transfer (FRET) using the secondary antibodies Alexa Fluor-488 and Alexa Fluor-594 as donor/acceptor pairs. b-e, FRET experiments on HaCaT cells utilizing antibodies against (b) ITGβ1and THY1 (b), ITGβ1 and α-catenin (c), α-catenin and ITGβ1 (d), and α-catenin and THY1 (e). f, Electron donor control for FRET experiments utilizing single antibodies (donor) and secondary antibodies alone (acceptor). g, Secondary antibody controls show no fluorescence in the absence of primary antibodies. h, Control for FRET utilizing distant electron donor/acceptor pairs ITGβ1 and Ki-67. i. High-magnification confocal image of non-confluent THY1-OE (THY1-overexpressed) keratinocytes immunostained against THY1 and α-catenin (n = 3 independent cultures per treatment). Panels on the right show single fluorescent channels. j, Whole lysate proteins isolated from WT and Thy1-/- dorsal skins immunoblotted against α-catenin and β-actin (n = 3 pooled mice of each). Scale bars, 10 μm (b,h) or 20 μm (i).
Extended Data Fig. 5 Dissecting cell-cell vs. cell-matrix mechanisms of THY1.
a, Immunostaining of active YAP in single HaCaT cells silenced for THY1 and co-treated with ITGβ1 blocking antibody, YAP inhibitor verteporfin or SRC inhibitor dasatinib. b, Percentage active YAP+ single cells (n = three independent cultures). c, qPCR for YAP targets in confluent cells: shSCR (Ctrl), shTHY1 and shTHY1 treated with either ITGβ1 blocking antibody, YAP inhibitor verteporfin or SRC inhibitor dasatinib. Values were normalized to RPLP0 (n = 3 independent cultures, analyzed in triplicates). d, Immunostaining of proliferation marker MCM2 in single HaCaT cells silenced for THY1 and co-treated with ITGβ1 blocking antibody, verteporfin or dasatinib. e, Percentage of MCM2+ single cells (n = three independent cultures). f, Relative number active YAP+ cells in the suprabasal layer of dorsal skins from WT and Thy1-/- mice (n = 5 mice). g, Percentage of active ITGβ1+ cells in the suprabasal layer of dorsal skins from WT and Thy1-/- mice (n = 3 mice). All images and quantifications are representative of at least n = 3 mice per genotype or 3 independent cultures per group, unless indicated otherwise. Error bars indicate mean ± s.e.m. Scale bars, 20 μm (a,d).
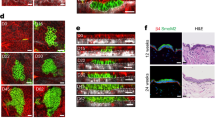
Extended Data Fig. 6 Thy1-/- mice display enhanced repair and regeneration.
a, Wound contraction at day 1 post wound infliction (PWI) (n = 7 mice). b,c, WT and Thy1-/- wounds imaged at day 7 (b) and day 18 (c) PWI. Dotted lines demarcate wound border. Asterisks denote areas of mechanical damage from epidermis/dermis separation. d, Immunostaining against active YAP and Ki-67 in the WT and Thy1-/- wound site at 18 days PWI. e-h, Active YAP expression in dorsal wounds after 3 days (e) and 7 days (f) PWI, or MCM2 expression after 3 days (g) and 7 days (h) PWI. i-k, Quantifications for percentages of active YAP+ (i) and MCM2+ (j) cells in the suprabasal layer, and number of cells (k) in the basal layer (n = 5 mice). l, Immunoblot of active YAP and β-actin in separated epidermal and dermal fractions from adult (P56) WT and Thy1-/- dorsal skins. m, Number of active YAP+ dermal cells throughout the wound healing process (n = 5 mice). n, Immunoblot of active YAP and β-actin in separated epidermal and dermal fractions from WT and Thy1-/- dorsal skins at 7 days PWI. o, Densitometry of active YAP signal (relating to panel n) (n = 3 pooled mice per group). p, qPCR for TGFβ1, SMAD2, SSMAD7 and p21 mRNA in WT and Thy1-/- dorsal skins at 7 days PWI. Values were normalized to RPLP0 (n = 3 mice, analyzed in triplicates). q, H&E images of WT and Thy1-/- dorsal wounds at 7 days PWI. Dashed black lines demarcate epidermis/dermis border. r, Regenerated Thy1-/- HFs (white arrowheads) immunostained against MCM2 and KRT15. s. Maximum projection images of WT and Thy1-/- wounds at 30 days PWI. t. Immunostaining against non-phosphorylated (active) β-catenin at day 7 PWI. u, qPCR against CTGF, SHH, WNT10a, WNT10b, WNT4 and WNT3 at day 12 PWI. Values were normalized to RPLP0 (n = 3 mice, analyzed in triplicates). All images are representative of at least n = 6 mice of each genotype, unless otherwise indicated. Error bars indicate mean ± s.e.m. Scale bars, 20 μm (d), 50 μm (e,f,g,h,t), 100 μm (r), 200 μm (q,s), 500 μm (b,c).
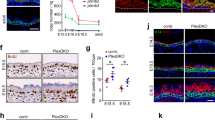
Extended Data Fig. 7 Effects of Thy1 loss on various cell types during wounding.
a, Confocal images of the WT and Thy1-/- wound beds at 3 days post wound infliction (PWI) immunostained against T cell marker CD3. White dotted lines demarcate epidermis/dermis. b, Flow cytometry on wounds resected at day 3 PWI gated for CD45-APC+ cells (n = 3 pooled mice of each genotype). c,d., Immunoblots against NFκB p65 (c) and IκBα (d) in epidermal and dermal cells of WT and Thy1-/- telogenic (P56) dorsal skins. e, Dermis of adult WT and Thy1-/- mice after 7 days PWI stained for MCM2 and the dermal fibroblast marker PDGFRα. f., Percentage of proliferating papillary and reticular dermal cells. g,h, Immunostaining against Vimentin and Ki-67 (g), and CD31 and Ki-67 (h) in wound site dermis at day 3 PWI. i, Immunostaining against CD31 and Ki-67 in wound site dermis at 7 days PWI. j, Relative numbers of proliferating CD31+ cells at 3 and 7 days PWI in WT and Thy1-/- mice (n = 3 mice). All images and quantifications are representative of at least n = 3 mice of each genotype, unless otherwise indicated. Quantifications were performed on ≥ 60 fields of view ranging from 100-200μm. Western blots were performed on pooled proteins from n = 3 mice per genotype. Error bars indicate mean ± s.e.m. Scale bars, 50 μm (a,e,g,h,i).
Extended Data Fig. 8 Inhibition of YAP abrogates Thy1-/–dependent phenotypes.
a, WT and Thy1-/- dorsal skins treated with 2.5% DMSO, and Thy1-/- treated with verteporfin (vert) at 36 h post BrdU (n = 9 mice per treatment). b, Number of BrdU+ cells from WT (DMSO-treated), Thy1-/- (DMSO-treated) and Thy1-/- (Vert-treated) mice (n = 9 mice per treatment). c, Schematic representation of YAP inhibition and dorsal wound infliction. d, Photographs of WT dorsal wounds treated with DMSO or Vert at day 7 post wound infliction (PWI). e, Re-epithelialization dynamics of WT skins. Percentage of wound coverage was calculated versus original wound size (n = 4 mice). f, Immunostaining against MCM2 and ITGβ4 (CD104) in dorsal wounds from WT (DMSO), Thy1-/- (DMSO) and Thy1-/- (Vert) mice. g, Percentage of MCM2+ suprabasal cells in treated mice (n = 4 mice). h, WT (DMSO), Thy1-/- (DMSO) and Thy1-/- (Vert) treated dorsal skins immunostained for MCM2 at day 7 PWI. i. Number of de novo hair follicles in the dorsal wound bed from WT (DMSO), Thy1-/- (DMSO) and Thy1-/- (Vert) mice at 7 days PWI (n = 4 mice). j. Schematic representation of ITGβ1 inhibition and dorsal wound infliction. k, WT dorsal skin treated with IgG or ITGβ1 antibody (ITGβ1 Ab) immunostained for active YAP and Ki-67 at day 7 PWI. l, Relative percentage active YAP+ cells in suprabasal layer from WT and Thy1-/- mice treated with IgG or ITGβ1 Ab at day 0 PWI (n = 4 mice). m. Dorsal skin sections from WT and Thy1-/- mice treated with IgG or ITGβ1 Ab immunostained for active YAP and Ki-67 at day 7 PWI. n-o. Quantifications of active YAP+ (n) and Ki-67+ (o) basal cells from WT and Thy1-/- mice treated with IgG or ITGβ1 Ab at day 7 PWI (n = 3 mice). p-q, Quantifications of active YAP+ (p) and Ki-67+ (q) suprabasal cells from WT and Thy1-/- mice treated with IgG or ITGβ1 antibodies at day 7 PWI (n = 3 mice). All images and quantifications are representative of at least n = 6 mice per genotype and treatment, unless otherwise stated. Error bars indicate mean ± s.e.m. Scale bars, 50 μm (a,f,h,k,m).
Supplementary information
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Sedov, E., Koren, E., Chopra, S. et al. THY1-mediated mechanisms converge to drive YAP activation in skin homeostasis and repair. Nat Cell Biol 24, 1049–1063 (2022). https://doi.org/10.1038/s41556-022-00944-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-022-00944-6
This article is cited by
-
Cellular and molecular mechanisms of skin wound healing
Nature Reviews Molecular Cell Biology (2024)
-
Reproducible strategy for excisional skin-wound-healing studies in mice
Nature Protocols (2024)
-
Insights into recent findings and clinical application of YAP and TAZ in cancer
Nature Reviews Cancer (2023)