Abstract
Piwi-interacting RNAs (piRNAs) are predominantly expressed in germ cells and function in gametogenesis in various species. However, Piwi-deficient female mice are fertile and mouse oocytes express a panel of small RNAs that do not appear to be widely representative of mammals. Thus, the function of piRNAs in mammalian oogenesis remains largely unclear. Here, we generated Piwil1- and Mov10l1-deficient golden hamsters and found that all female and male mutants were sterile, with severe defects in embryogenesis and spermatogenesis, respectively. In Piwil1-deficient female hamsters, the oocytes and embryos displayed aberrant transposon accumulation and extensive transcriptomic dysregulation, and the embryos were arrested at the two-cell stage with impaired zygotic genome activation. Moreover, PIWIL1-piRNAs exert a non-redundant function in silencing endogenous retroviruses in the oocytes and embryos. Together, our findings demonstrate that piRNAs are indispensable for generating functional germ cells in golden hamsters and show the value of this model species for piRNA studies in gametogenesis, especially those related to female infertility.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The deep-sequencing data have been deposited at the National Center for Biotechnology Information Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) database under accession number GSE169528. All other data supporting the findings of this study are available from the corresponding authors on reasonable request. Source data are provided with this paper.
References
Roovers, E. F. et al. Piwi proteins and piRNAs in mammalian oocytes and early embryos. Cell Rep. 10, 2069–2082 (2015).
Watanabe, T. et al. Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Gene Dev. 20, 1732–1743 (2006).
Yang, Q. et al. Single-cell CAS-seq reveals a class of short PIWI-interacting RNAs in human oocytes. Nat. Commun. 10, 3389 (2019).
Vagin, V. V. et al. A distinct small RNA pathway silences selfish genetic elements in the germline. Science 313, 320–324 (2006).
Lau, N. C. et al. Characterization of the piRNA complex from rat testes. Science 313, 363–367 (2006).
Grivna, S. T., Beyret, E., Wang, Z. & Lin, H. F. A novel class of small RNAs in mouse spermatogenic cells. Gene Dev. 20, 1709–1714 (2006).
Girard, A., Sachidanandam, R., Hannon, G. J. & Carmell, M. A. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442, 199–202 (2006).
Aravin, A. et al. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442, 203–207 (2006).
Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. & Zamore, P. D. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 20, 89–108 (2019).
Czech, B. et al. piRNA-guided genome defense: from biogenesis to silencing. Annu. Rev. Genet. 52, 131–157 (2018).
Iwasaki, Y. W., Siomi, M. C. & Siomi, H. PIWI-interacting RNA: its biogenesis and functions. Annu. Rev. Biochem. 84, 405–433 (2015).
Ross, R. J., Weiner, M. M. & Lin, H. F. PIWI proteins and PIWI-interacting RNAs in the soma. Nature 505, 353–359 (2014).
Cox, D. N. et al. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Gene Dev. 12, 3715–3727 (1998).
Li, C. J. et al. Collapse of germline piRNAs in the absence of Argonaute3 reveals somatic piRNAs in flies. Cell 137, 509–521 (2009).
Houwing, S., Berezikov, E. & Ketting, R. F. Zili is required for germ cell differentiation and meiosis in zebrafish. EMBO J. 27, 2702–2711 (2008).
Houwing, S. et al. A role for Piwi and piRNAs in germ cell maintenance and transposon silencing in zebrafish. Cell 129, 69–82 (2007).
Cox, D. N., Chao, A. & Lin, H. F. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development 127, 503–514 (2000).
Carmell, M. A. et al. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev. Cell 12, 503–514 (2007).
Kuramochi-Miyagawa, S. et al. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131, 839–849 (2004).
Deng, W. & Lin, H. F. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev. Cell 2, 819–830 (2002).
Watanabe, T. et al. MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev. Cell 20, 364–375 (2011).
Huang, H. Y. et al. piRNA-associated germline nuage formation and spermatogenesis require mitoPLD profusogenic mitochondrial-surface lipid signaling. Dev. Cell 20, 376–387 (2011).
Zheng, K. et al. Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc. Natl Acad. Sci. USA 107, 11841–11846 (2010).
Frost, R. J. A. et al. MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc. Natl Acad. Sci. USA 107, 11847–11852 (2010).
Flemr, M. et al. A retrotransposon-driven Dicer isoform directs endogenous small interfering RNA production in mouse oocytes. Cell 155, 807–816 (2013).
Watanabe, T. et al. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453, 539–543 (2008).
Tam, O. H. et al. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453, 534–538 (2008).
Hirose, M. & Ogura, A. The golden (Syrian) hamster as a model for the study of reproductive biology: past, present, and future. Reprod. Med Biol. 18, 34–39 (2019).
Ishino, K. et al. Hamster PIWI proteins bind to piRNAs with stage-specific size variations during oocyte maturation. Nucleic Acids Res. 49, 2700–2720 (2021).
Fan, Z. Q. et al. Efficient gene targeting in golden Syrian hamsters by the CRISPR/Cas9 system. PLoS ONE 9, e109755 (2014).
Hirose, M. et al. Acrosin is essential for sperm penetration through the zona pellucida in hamsters. Proc. Natl Acad. Sci. USA 117, 2513–2518 (2020).
Hustedt, N. & Durocher, D. The control of DNA repair by the cell cycle. Nat. Cell Biol. 19, 1–9 (2016).
Gu, B., Posfai, E. & Rossant, J. Efficient generation of targeted large insertions by microinjection into two-cell-stage mouse embryos. Nat. Biotechnol. 36, 632–637 (2018).
Zenker, J. et al. A microtubule-organizing center directing intracellular transport in the early mouse embryo. Science 357, 925–928 (2017).
Vourekas, A. et al. The RNA helicase MOV10L1 binds piRNA precursors to initiate piRNA processing. Gene Dev. 29, 617–629 (2015).
Zheng, K. & Wang, P. J. Blockade of pachytene piRNA biogenesis reveals a novel requirement for maintaining post-meiotic germline genome integrity. PLoS Genet. 8, e1003038 (2012).
Rodriguez-Terrones, D. & Torres-Padilla, M. E. Nimble and ready to mingle: transposon outbursts of early development. Trends Genet. 34, 806–820 (2018).
Gunawardane, L. S. et al. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315, 1587–1590 (2007).
Brennecke, J. et al. Discrete small RNA-generating loci as master regulators of transposon activity in Drosophila. Cell 128, 1089–1103 (2007).
Schulz, K. N. & Harrison, M. M. Mechanisms regulating zygotic genome activation. Nat. Rev. Genet. 20, 221–234 (2019).
Harvey, A. J. Mitochondria in early development: linking the microenvironment, metabolism and the epigenome. Reproduction 157, R159–R179 (2019).
Taborska, E. et al. Restricted and non-essential redundancy of RNAi and piRNA pathways in mouse oocytes. PLoS Genet. 15, e1008261 (2019).
Su, R. et al. Global profiling of RNA-binding protein target sites by LACE-seq. Nat. Cell Biol. 23, 664–675 (2021).
Halbach, R. et al. A satellite repeat-derived piRNA controls embryonic development of Aedes. Nature 580, 274–277 (2020).
Rouget, C. et al. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 467, 1128–1132 (2010).
Loubalova, Z. et al. Formation of spermatogonia and fertile oocytes in golden hamsters requires piRNAs. Nat. Cell Biol. https://doi.org/10.1038/s41556-021-00746-2 (2021).
Hasuwa, H. et al. Production of functional oocytes requires maternally expressed PIWI genes and piRNAs in golden hamsters. Nat. Cell Biol. https://doi.org/10.1038/s41556-021-00745-3 (2021).
Zhang, Y. et al. Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol. Cell 72, 1021–1034 (2018).
Vanacker, J. et al. Enzymatic isolation of human primordial and primary ovarian follicles with Liberase DH: protocol for application in a clinical setting. Fertil. Steril. 96, 379–383 (2011).
Cong, L. et al. Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 (2013).
Yang, Q. et al. Single-cell CAS-seq reveals a class of short PIWI-interacting RNAs in human oocytes. Nat. Commun. 10, 3389 (2019).
Anderson, L. K., Reeves, A., Webb, L. M. & Ashley, T. Distribution of crossing over on mouse synaptonemal complexes using immunofluorescent localization of MLH1 protein. Genetics 151, 1569–1579 (1999).
Alavattam, K. G., Abe, H., Sakashita, A. & Namekawa, S. H. Chromosome spread analyses of meiotic sex chromosome inactivation. Methods Mol. Biol. 1861, 113–129 (2018).
Picelli, S. et al. Smart-seq2 for sensitive full-length transcriptome profiling in single cells. Nat. Methods 10, 1096–1098 (2013).
Picelli, S. et al. Tn5 transposase and tagmentation procedures for massively scaled sequencing projects. Genome Res. 24, 2033–2040 (2014).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Jung, I., Park, J. C. & Kim, S. piClust: a density based piRNA clustering algorithm. Comput. Biol. Chem. 50, 60–67 (2014).
Stuart, T. et al. Comprehensive integration of single-cell data. Cell 177, 1888–1902 (2019).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Flynn, J. M. et al. RepeatModeler2 for automated genomic discovery of transposable element families. Proc. Natl Acad. Sci. USA 117, 9451–9457 (2020).
Altschul, S. F. et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25, 3389–3402 (1997).
Zhi, D., Raphael, B. J., Price, A. L., Tang, H. & Pevzner, P. A. Identifying repeat domains in large genomes. Genome Biol. 7, R7 (2006).
Li, X. Z. G. et al. An ancient transcription factor initiates the burst of piRNA production during early meiosis in mouse testes. Mol. Cell 50, 67–81 (2013).
Chan, P. P. & Lowe, T. M. GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 37, D93–D97 (2009).
Acknowledgements
We thank all members of L. Wu’s and J. Li’s laboratories for their discussion and comments on this project; M. Tong and W. Shum for their comments and suggestions; Z. Li for bioinformatics analyses and Y. Song for library construction; Y. Xu for his assistance with high-performance computing; staff at the HPC storage and network service platform of SIBCB for supplying the computing resources; H. Siomi (Keio University School of Medicine, Tokyo, Japan) for sharing unpublished data; and Y. Huang, M. Liu and Y. Yu for their comments on the manuscript. This work was supported by the following funding: the National Natural Science Foundation of Jiangsu Province (BE2019730) to J.L.; the National Key R&D Program of China (2017YFA0504401), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB19040102) and the National Natural Science Foundation of China (31970607 and 31470781) to L.W.; the National Basic Research (973) Program of China (2009CB941700), Natural Science Foundation (SKLRM-2021B5) of State Key Lab of Reproductive Medicine and the National Natural Science Foundation of China (31171443) to J.L.; the National Key Research and Development Program of China (2018YFC1003800), the National Natural Science Foundation of China (31871507, 31471351 and 31271538), the National Basic Research (973) Program of China (2014CB943200 and 2013CB945500) and the National Natural Science Foundation of Jiangsu Province (BK20140061) to Y.-Q.S.
Author information
Authors and Affiliations
Contributions
L.W. and J.L. conceived and designed the study. J.L. and W.Z. conceived and developed the methodology for genome editing of golden hamsters. W.Z. generated the Piwil1 and Mov10l1-deficient golden hamsters with the help of J.Z. and Y.L.; Q.C., M.L., X.L., Y.Z., S.G. and Y.-Q.S. analysed the phenotype of Piwil1 mutants and Z.Z., F.D. and H.F. analysed the phenotype of Mov10l1 mutants, with the help of A.S. and A.Z.; H.Z. conducted small-RNA- and RNA-sequencing analysis. L.H. conducted PIWIL1 IP-seq and oxidation-seq. F.Z. performed bioinformatics analyses with the help of Y.X. and W.X.; H.Z., F.Z., J.L. and L.W. interpreted the data of the experiments and wrote the manuscript. J.L. is the lead corresponding author.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cell Biology thanks René Ketting, P. Jeremy Wang and William Theurkauf for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Generation of Tyr, Piwil1 and Mov10l1 mutant golden hamsters.
(a) Strategy for the generation of albino (Tyr mutants) golden hamsters. Two-cell embryos were injected with CRISPR/Cas9 and sgRNAs and transferred to naturally pregnant recipients. (b) Diagram of the golden hamster Tyr gene and the resulting Tyr mutants. The sgRNA was designed to target Exon 1. PAM, protospacer adjacent motif. (c) Production of albino golden hamster lines. Albino golden hamster lines were established by 7 mutant founders. (d) The live birth rate after transfer of injected 1-cell or 2-cell embryos. Data are presented as mean values ± s.e.m. n = 3 or n = 5 biologically independent experiments for PN injection or 2 C injection, respectively. Statistical analysis was performed using unpaired two-tailed t-test. Statistical data are provided in the source data. (e) Strategy for the generation of Piwil1 mutant golden hamsters by CRISPR/Cas9. (f) Diagram of wild type, mutant1- and mutant2-PIWIL1 protein. Both mutant1 and mutant2 are frameshift variants. (g-h) Immunostaining shows loss of PIWIL1 expression in MII oocytes (g) and 2-cell embryos (h) of Piwil1m1/m1. Nuclei were stained with DAPI. Scale bars = 50 μm. The experiments were independently repeated twice with similar results. (i) Structure of golden hamster the Mov10l1 gene and generation of Mov10l1 mutants (Mov10l1ins1/ins1). The Mov10l1ins1/ins1 contained one thymine (T) insertion in exon 2. (j) Diagram of wild-type and MOV10L1 mutant protein. Mov10l1ins1/ins1 caused a frameshift that generated a premature stop codon in Mov10l11 mRNAs.
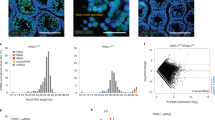
Extended Data Fig. 2 Piwil1-deficient oocytes show no obvious phenotypic abnormality but lack developmental competence for early embryos.
(a) PAS staining of wild type and Piwil1m1/m1 ovarian sections. (b) The average numbers of ovulated oocytes collected from wild-type and Piwil1m1/m1 females, as determined by a superovulation assay. Data are presented as mean values ± s.e.m. p-value = 0.2 by unpaired two-tailed t-test (n = 10 superovulated golden hamsters per group). (c) Immunofluorescence staining of the MII oocytes and early embryos using Actin- and Tubulin-specific antibodies. Nuclei were stained with DAPI. The results show normal morphology of spindles in Piwil1-deficient MII oocytes and the absence of the microtubule bridge (white arrow) in maternal Piwil1-deficient embryos. Piwil1m1/m1 indicates Piwil1-deficient oocytes or maternal Piwil1-deficient embryos. (d) Representative images of in vitro cultured embryos obtained from wild-type and Piwil1m1/m1 oocytes fertilized in vivo with wild-type sperm at 9, 33, 44, and 52 h.p.e.a. Red arrows, pronuclei. (e) Representative images of embryos collected from the oviducts of wild-type and Piwil1m2/m2 females at 52 h.p.e.a. The assays in a and c-d were performed twice, with similar results obtained. Statistical data are provided in the source data.
Extended Data Fig. 3 Spermatogenesis defects in Piwil1- and Mov10l1-deficient golden hamsters.
(a) Comparison of the testes from 8-week-old wild-type, Piwil1m1/m1, Piwil1m2/m2 and Mov10l1ins1/ins1 golden hamsters. (b) The testis/body weight ratios. Testes were collected from 8-week-old wild-type, Piwil1w/m1, Piwil1m1/m1 and Mov10l1ins1/ins1 golden hamsters. Data are presented as mean values ± s.e.m. Statistical analysis was performed using unpaired two-tailed t-test. n = 4 Piwil1 mutants or n = 5 Mov10l1 mutants. (c-d) Periodic acid-Schiff (PAS) staining of adult caput epididymis (c), cauda epididymis (d). (e) H&E staining of WT and Mov10l1ins1/ins1 testes at 8, 14, 28, and 42 days post partum (d.p.p.). The assays in c, d and e were independently repeated twice and showed similar results. Statistical data are provided in the source data.
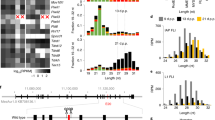
Extended Data Fig. 4 Sequence analysis and Ping-Pong signature of piRNAs in WT and Piwil1-deficient females.
(a) The piRNAs identified in oocytes and embryos collected from wild-type females showed three main peaks in their size distribution: 18–20-nucleotide (19-nucleotide piRNA), 22–24-nucleotide (23-nucleotide piRNA), and 28–30-nucleotide (29-nucleotide piRNA). All three piRNA populations preferentially carried a 5′ uracil (U). Oocytes and embryos collected from Piwil1m1/m1 females only expressed 19-nucleotide piRNAs, which showed a strong preference for a 5′ U. Piwil1m1/m1 indicates Piwil1-deficient oocytes or maternal Piwil1-deficient embryos. Data shown were average value of biological replicates at each time point: n = 3 (PF), 3 (SF), 3 (GV), 3 (MII), 3 (9 h.p.e.a.), 6 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 3 (52 h.p.e.a.) for WT; n = 2 (PF), 2 (SF), 2 (GV), 2 (MII), 3 (9 h.p.e.a.), 2 (33 h.p.e.a), 4 (44 h.p.e.a) or 4 (52 h.p.e.a) for Piwil1 mutants. (b) Sequence analysis of piRNAs bound to PIWIL1 and PIWIL3 in MII oocytes. (c) The 5’-5’ overlap between sense- and antisense strands of TE-derived piRNAs bound to PIWIL1 or PIWIL3 were analyzed for the presence of Ping-Pong signatures. The number of pairs of piRNA reads at each position is plotted. Significance of 10-nucleotide overlap (‘Ping-Pong’) was determined based on Z score; a Z score > 1.96 corresponds to p-value < 0.05.
Extended Data Fig. 5 Characterization of small RNAs in WT and Piwil1 mutants.
(a) Heat maps show the expression level (RPKM) of top piRNA clusters, which generated >90% of the unique mapped piRNAs in each oocyte and embryo at different developmental stages. Piwil1m1/m1 indicates Piwil1-deficient oocytes or maternal Piwil1-deficient embryos. PF, primary follicle stage oocyte; SF, secondary follicle stage oocyte; GV, germinal vesicle stage oocyte; MII, metaphase II oocyte. (b) The relative abundances of 23-nucleotide, 29-nucleotide and 19-nucleotide piRNAs among total piRNAs (a) in each cluster are shown. Almost all of the piRNA clusters that produce PIWIL1 23-nucleotide and 29-nucleotide piRNAs are identical, while several piRNA clusters uniquely produced PIWIL3 19-nucleotide piRNAs. (c) The ratio of piRNAs with identical 5’ ends (up) and 3’ ends (bottom) among PIWIL1 23-nucleotide, PIWIL1 29-nucleotide and PIWIL3 19-nucleotide piRNAs. Only uniquely mapped piRNAs are calculated in (a) and (b). Data shown in a-c were average value of biological replicates at each time point: n = 3 (PF), 3 (SF), 3 (GV), 3 (MII), 3 (9 h.p.e.a.), 6 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 3 (52 h.p.e.a.) for WT; n = 2 (PF), 2 (SF), 2 (GV), 2 (MII), 3 (9 h.p.e.a.), 2 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 4 (52 h.p.e.a.) for Piwil1 mutants. (d) Differential analysis of miRNA expression during oogenesis and early embryo development. miRDeep2 was used for de novo miRNA identification. m, Piwil1-deficient oocytes or maternal Piwil1-deficient embryos; w, WT oocytes or embryos. (e) Sequence analysis of piRNAs expressed in testes. The 28–31-nucleotide piRNAs found in wild-type testes and the remaining 26–28-nucleotide piRNAs in Piwil1m1/m1 testes both showed a strong preference for 5′ U. (f) Composition of small RNAs in wild-type and Piwil1m1/m1 testes with or without NaIO4 oxidation treatment. The small RNA counts were normalized by exogenous spike-in. (g) Size distribution of PIWIL1-bound piRNAs in wild-type and Piwil1m1/m1 testes immunoprecipitated with PIWIL1-specific antibody. Rabbit non-specific immunoglobulin G (IgG) served as a negative control. The small RNA counts were normalized by exogenous spike-in. (h) Sequence analysis of PIWIL1-piRNAs expressed in testis.
Extended Data Fig. 6 Genomic distribution of piRNAs at different stages.
(a) Genomic annotation of piRNA counts of different sizes identified in oocytes at the primary follicle stage through embryos at 52 h.p.e.a in wild-type and Piwil1m1/m1 golden hamsters. PF, primary follicle stage oocyte; SF, secondary follicle stage oocyte; GV, germinal vesicle stage oocyte; MII, metaphase II oocyte; 1 C, one-cell embryo; 2 C, two-cell embryo; 4 C, four-cell embryo. Piwil1m1/m1 indicates Piwil1-deficient oocytes or maternal Piwil1-deficient embryos. (b) Bar graphs show the distribution of piRNAs generated from different genomic regions. PIWIL1 29-nucleotide piRNAs, PIWIL1 23-nucleotide piRNA, and PIWIL3 19-nucleotide piRNAs were designated based on their location on the genome (piRNA cluster) and length, and were analyzed separately. Histograms left of the vertical dashed line show different families of repeat elements; histograms right of the vertical dashed line show the gene-related regions. The combination of Low complexity, Simple repeat, and Satellite is designated as LSS. Piwil1m1/m1 indicates Piwil1-deficient oocytes or maternal Piwil1-deficient embryos. Data shown a-b were average value of biological replicates at each time point: n = 3 (PF), 3 (SF), 3 (GV), 3 (MII), 3 (9 h.p.e.a.), 6 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 3 (52 h.p.e.a.) for WT; n = 2 (PF), 2 (SF), 2 (GV), 2 (MII), 3 (9 h.p.e.a.), 2 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 4 (52 h.p.e.a) for Piwil1 mutants.
Extended Data Fig. 7 TE expression levels during oogenesis and embryo development.
(a) Volcano plots show the differentially expressed TEs between two adjacent stages during oogenesis and embryo development in wild-type golden hamsters. The highly significant up- or down-regulated TEs (≥ 2 folds; Welch two sample t-test, p-value < 0.05) are indicated in red or blue, respectively, with TE numbers shown at the top. PF, primary follicle stage oocyte; SF, secondary follicle stage oocyte; GV, germinal vesicle stage oocyte; MII, metaphase II oocyte. (b) Heat map of up-regulated TE expression levels in oocytes and embryos derived from WT and Piwil1-deficient females. The highly significant up-regulated TEs (≥ 2 folds) in Piwil1-deficient oocytes and maternal Piwil1-deficient embryos compared to wild-type oocytes and embryos at PF, SF, GV, MII, and 9 h.p.e.a. are plotted. Piwil1m1/m1 indicates Piwil1-deficient oocytes or maternal Piwil1-deficient embryos. Data shown were average value of biological replicates at each time point: n = 3 (PF), 3 (SF), 3 (GV), 3 (MII), 3 (9 h.p.e.a.), 6 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 3 (52 h.p.e.a.) for WT; n = 2 (PF), 2 (SF), 2 (GV), 2 (MII), 3 (9 h.p.e.a.), 2 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 4 (52 h.p.e.a.) for Piwil1 mutants.
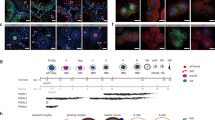
Extended Data Fig. 8 Anti-sense piRNAs were enriched with up-regulated TEs.
(a-b) The bar graph shows the expression level (RPKM) of piRNAs mapped to the sense (gray) or anti-sense (red) sequences of each TE family in GV oocytes (a) and 1-cell embryos at 9 h.p.e.a. (b). Different TE family-derived piRNAs are designated based on their location on the genome (piRNA cluster) and length, and are plotted. The significantly up-regulated TE families are listed with log2 fold change in expression level between Piwil1 mutant versus wild-type GV oocytes or maternal Piwil1-deficient and WT zygotes. The fold change level is indicated by differences in orange hue in the heat map. Data shown in a are average value from 3 biological replicates for WT and 2 biological replicates for mutants. Data shown in b are average values from 3 biological replicates for WT or Piwil1 mutants. (c) Examples of PIWIL1-piRNA and PIWIL3-piRNA distribution in ERV families that were up-regulated in Piwil1m1/m1 MII oocytes. The expression levels (RPKM) represent the normalized number of all mapped piRNAs with the same 5’ end at each position. The dotted boxes indicate the Ping-Pong signal between the adjacent sense and antisense piRNAs. (d) The 5’-5’ overlap between piRNA sense- and antisense strands were analyzed for the presence of Ping-Pong signatures. The number of pairs of piRNA reads at each position is plotted. Significance of 10-nucleotide overlap (‘Ping-Pong’) was determined based on Z score; a Z score > 1.96 corresponds to p-value < 0.05. These PIWIL1-piRNAs (23-nucleotide and 29-nucleotide piRNAs) and PIWIL3-piRNAs (19-nucleotide piRNAs) in panel c and d are designated based on their location on the genome (piRNA cluster) and length. Data shown in c-d are average value from 3 biological replicates for WT and 2 biological replicates for Piwil1 mutants.
Extended Data Fig. 9 Gene ontology (GO) enrichment analysis of differentially expressed genes in maternal Piwil1-deficient embryos.
Top-ranking GO terms (biological processes) of differential expressed genes in maternal Piwil1-deficient embryos at 9, 33, 44 and 52 h.p.e.a.
Extended Data Fig. 10 ZGA is impaired in maternal Piwil1-deficient embryos.
(a-b) Heat maps of gene expressions in oocytes and embryos derived from WT and Piwil1-deficient females. Only the genes which were up-regulated in wild-type embryos from 9 h.p.e.a. to 33 h.p.e.a. (a) or 33 h.p.e.a. to 44 h.p.e.a. (b) are shown. The up-regulation of gene expression in early embryogenesis of wild-type embryos was barely detectable in maternal Piwil1-deficient embryos. Data shown were average value of biological replicates at each time point: n = 3 (PF), 3 (SF), 3 (GV), 3 (MII), 3 (9 h.p.e.a.), 6 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 3 (52 h.p.e.a.) for WT; n = 2 (PF), 2 (SF), 2 (GV), 2 (MII), 3 (9 h.p.e.a.), 2 (33 h.p.e.a.), 4 (44 h.p.e.a.) or 4 (52 h.p.e.a.) for Piwil1 mutants. PF, primary follicle stage oocyte; SF, secondary follicle stage oocyte; GV, germinal vesicle stage oocyte; MII, metaphase II oocyte. Piwil1m1/m1 indicates Piwil1-deficient oocytes or maternal Piwil1-deficient embryos. (c) Degradation of some maternal mRNAs is inhibited during the development of the Piwil1-deficient MII oocytes to maternal Piwil1-deficient 1-cell embryos at 9 h.p.e.a. and the maternal Piwil1-deficient 1-cell embryos at 9 h.p.e.a. to 2-cell embryos at 33 h.p.e.a. X- and Y-axes represent the log2 fold change of gene expression between adjacent developmental stages in WT (X-axis) or (maternal) Piwil1-deficient oocytes (embryos) (Y-axis), respectively.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Supplementary Tables 1–6
Supplementary Table 1: list of oligonucleotide sequences. Supplementary Table 2: sequencing data summary of small- and long-RNAs. Supplementary Table 3: the top piRNA clusters that generated >90% of the unique mapped piRNAs in each oocyte and embryo at different developmental stages are shown. The expression level of unique mapped piRNAs generated by these top clusters in different samples were calculated. Supplementary Table 4: predicted miRNAs information and expression levels (RPM) in oocytes and early embryos derived from WT and Piwil1-deficient golden hamsters. Supplementary Table 5: consensus-TE expression levels in oocytes and early embryos derived from WT and Piwil1-deficient golden hamsters. Supplementary Table 6: gene expression levels in oocytes and early embryos derived from wild-type and Piwil1-deficient golden hamsters.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhang, H., Zhang, F., Chen, Q. et al. The piRNA pathway is essential for generating functional oocytes in golden hamsters. Nat Cell Biol 23, 1013–1022 (2021). https://doi.org/10.1038/s41556-021-00750-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-021-00750-6
This article is cited by
-
Divergent composition and transposon-silencing activity of small RNAs in mammalian oocytes
Genome Biology (2024)
-
Tissue-specific profiling of age-dependent miRNAomic changes in Caenorhabditis elegans
Nature Communications (2024)
-
Themes and variations on piRNA-guided transposon control
Mobile DNA (2023)
-
Exploring right ovary degeneration in duck and goose embryos by histology and transcriptome dynamics analysis
BMC Genomics (2023)
-
Human zygotic genome activation is initiated from paternal genome
Cell Discovery (2023)