Abstract
The memory of stresses experienced by parents can be passed on to descendants as a forecast of the challenges to come. Here, we discovered that the neuronal mitochondrial perturbation-induced systemic mitochondrial unfolded protein response (UPRmt) in Caenorhabditis elegans can be transmitted to offspring over multiple generations. The transgenerational activation of UPRmt is mediated by maternal inheritance of elevated levels of mitochondrial DNA (mtDNA), which causes the proteostasis stress within mitochondria. Furthermore, results from intercrossing studies using wild C. elegans strains further support that maternal inheritance of higher levels of mtDNA can induce the UPRmt in descendants. The mitokine Wnt signalling pathway is required for the transmission of elevated mtDNA levels across generations, thereby conferring lifespan extension and stress resistance to offspring. Collectively, our results reveal that the nervous system can transmit stress signals across generations by increasing mtDNA in the germline, enabling descendants to better cope with anticipated challenges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The accession numbers for the raw sequencing files and the processed data reported in this paper are NCBI GEO: GSE157031, and NCBI BioProject: PRJNA607689 and PRJNA727630. Previously published DNA-sequencing data that were used here are available under accession code PRJNA13758. The KEGG pathway database used in this study is available online (http://www.genome.jp/kegg/). All other data supporting the findings of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Rechavi, O. et al. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 158, 277–287 (2014).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Perez, M. F., Francesconi, M., Hidalgo-Carcedo, C. & Lehner, B. Maternal age generates phenotypic variation in Caenorhabditis elegans. Nature 552, 106–109 (2017).
Perez, M. F. & Lehner, B. Intergenerational and transgenerational epigenetic inheritance in animals. Nat. Cell Biol. 21, 143–151 (2019).
Skvortsova, K., Iovino, N. & Bogdanović, O. Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol. 19, 774–790 (2018).
Webster, A. K., Jordan, J. M., Hibshman, J. D., Chitrakar, R. & Baugh, R. L. Transgenerational effects of extended dauer diapause on starvation survival and gene expression plasticity in Caenorhabditis elegans. Genetics 210, 263–274 (2018).
Jobson, M. A. et al. Transgenerational effects of early life starvation on growth, reproduction, and stress resistance in Caenorhabditis elegans. Genetics 201, 201–212 (2015).
Baugh, L. R. & Day, T. Nongenetic inheritance and multigenerational plasticity in the nematode C. Elegans. eLife 9, e58498 (2020).
Wallace, D. C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Ann. Rev. Genet. 39, 359–407 (2005).
Shpilka, T. & Haynes, C. M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 19, 109–120 (2018).
Ma, C. et al. N6-methyldeoxyadenine is a transgenerational epigenetic signal for mitochondrial stress adaptation. Nat. Cell Biol. 21, 319–327 (2019).
Hibshman, J. D., Hung, A. & Baugh, L. R. Maternal diet and insulin-like signaling control intergenerational plasticity of progeny size and starvation resistance. PLoS Genet. 12, e1006396 (2016).
Jordan, J. M. et al. Insulin/IGF signaling and vitellogenin provisioning mediate intergenerational adaptation to nutrient stress. Curr. Biol. 29, 2380–2388 (2019).
Okimoto, R., Macfarlane, J. L., Clary, D. O. & Wolstenholme, D. R. The mitochondrial genomes of two nematodes, Caenorhabditis elegans and Ascaris suum. Genetics 130, 471–498 (1992).
Houtkooper, R. H. et al. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497, 451–457 (2013).
Martinus, R. D. et al. Selective induction of mitochondrial chaperones in response to loss of the mitochondrial genome. Eur. J. Biochem. 240, 98–103 (1996).
Yoneda, T. et al. Compartment-specific perturbation of protein handling activates genes encoding mitochondrial chaperones. J. Cell Sci. 117, 4055–4066 (2004).
Tian, Y. et al. Mitochondrial stress induces chromatin reorganization to promote longevity and UPRmt. Cell 165, 1197–1208 (2016).
Merkwirth, C. et al. Two conserved histone demethylases regulate mitochondrial stress-induced longevity. Cell 165, 1209–1223 (2016).
Zhu, D. et al. NuRD mediates mitochondrial stress–induced longevity via chromatin remodeling in response to acetyl-CoA level. Sci. Adv. 6, eabb2529 (2020).
Benedetti, C., Haynes, C. M., Yang, Y., Harding, H. P. & Ron, D. Ubiquitin-like protein 5 positively regulates chaperone gene expression in the mitochondrial unfolded protein response. Genetics 174, 229–239 (2006).
Nargund, A. M., Pellegrino, M. W., Fiorese, C. J., Baker, B. M. & Haynes, C. M. Mitochondrial import efficiency of ATFS-1 regulates mitochondrial UPR activation. Science 337, 587–590 (2012).
Nargund, A. M., Fiorese, C. J., Pellegrino, M. W., Deng, P. & Haynes, C. M. Mitochondrial and nuclear accumulation of the transcription factor ATFS-1 promotes OXPHOS recovery during the UPRmt. Mol. Cell 58, 123–133 (2015).
Durieux, J., Wolff, S. & Dillin, A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell 144, 79–91 (2011).
Berendzen, K. M. et al. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell 166, 1553–1563 (2016).
Lan, J. et al. Translational regulation of non-autonomous mitochondrial stress response promotes longevity. Cell Rep. 28, 1050–1062 (2019).
Higuchi-Sanabria, R., Frankino, P. A., Paul, J. W., Tronnes, S. U. & Dillin, A. A futile battle? Protein quality control and the stress of aging. Dev. Cell 44, 139–163 (2018).
Suomalainen, A. et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 10, 806–818 (2011).
Owusu-Ansah, E., Song, W. & Perrimon, N. Muscle mitohormesis promotes longevity via systemic repression of insulin signaling. Cell 155, 699–712 (2013).
Kang, G. M. et al. Mitohormesis in hypothalamic POMC neurons mediates regular exercise-induced high-turnover metabolism. Cell Metab. 33, 334–349 (2021).
Shao, L. W., Niu, R. & Liu, Y. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 26, 1182–1196 (2016).
Zhang, Q. et al. The mitochondrial unfolded protein response is mediated cell-non-autonomously by retromer-dependent Wnt signaling. Cell 174, 870–883 (2018).
Taylor, R. C. & Dillin, A. XBP-1 is a cell-nonautonomous regulator of stress resistance and longevity. Cell 153, 1435–1447 (2013).
Lin, Y. F. et al. Maintenance and propagation of a deleterious mitochondrial genome by the mitochondrial unfolded protein response. Nature 533, 416–419 (2016).
Tsang, W. Y. & Lemire, B. D. Mitochondrial genome content is regulated during nematode development. Biochem. Biophys. Res. Commun. 291, 8–16 (2002).
Haynes, C. M., Yang, Y., Blais, S. P., Neubert, T. A. & Ron, D. The matrix peptide exporter HAF-1 signals a mitochondrial UPR by activating the transcription factor ZC376.7 in C. elegans. Mol. Cell 37, 529–540 (2010).
Korswagen, H. C. et al. The Axin-like protein PRY-1 is a negative regulator of a canonical Wnt pathway in C. elegans. Genes Dev. 16, 1291–1302 (2002).
Narendra, D. P. et al. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 8, e1000298 (2010).
Silva Ramos, E. et al. Mitochondrial fusion is required for regulation of mitochondrial DNA replication. PLoS Genet. 15, e1008085 (2019).
Jones, B. A. & Fangman, W. L. Mitochondrial DNA maintenance in yeast requires a protein containing a region related to the GTP-binding domain of dynamin. Genes Dev. 6, 380–389 (1992).
Bratic, I. et al. Mitochondrial DNA level, but not active replicase, is essential for Caenorhabditis elegans development. Nucleic Acids Res. 37, 1817–1828 (2009).
Maydan, J. S., Lorch, A., Edgley, M. L., Flibotte, S. & Moerman, D. G. Copy number variation in the genomes of twelve natural isolates of Caenorhabditis elegans. BMC Genomics 11, 62 (2010).
Andersen, E. C. et al. Chromosome-scale selective sweeps shape Caenorhabditis elegans genomic diversity. Nat. Genet. 44, 285–290 (2012).
Pellegrino, M. W. et al. Mitochondrial UPR-regulated innate immunity provides resistance to pathogen infection. Nature 516, 414–417 (2014).
Tan, M. W., Mahajan-Miklos, S. & Ausubel, F. M. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl Acad. Sci. USA 96, 715–720 (1999).
Mousseau, T. A. & Fox, C. W. The adaptive significance of maternal effects. Trends Ecol. Evol. 13, 403–407 (1998).
Öst, A. et al. Paternal diet defines offspring chromatin state and intergenerational obesity. Cell 159, 1352–1364 (2014).
Klosin, A., Casas, E., Hidalgo-Carcedo, C., Vavouri, T. & Lehner, B. Transgenerational transmission of environmental information in C. elegans. Science 356, 320–323 (2017).
Dias, B. G. & Ressler, K. J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Nat. Neurosci. 17, 89–96 (2014).
Moore, R. S., Kaletsky, R. & Murphy, C. T. Piwi/PRG-1 argonaute and TGF-β mediate transgenerational learned pathogenic avoidance. Cell 177, 1827–1841 (2019).
Posner, R. et al. Neuronal small RNAs control behavior transgenerationally. Cell 177, 1814–1826 (2019).
Mengel-From, J. et al. Mitochondrial DNA copy number in peripheral blood cells declines with age and is associated with general health among elderly. Hum. Genet. 133, 1149–1159 (2014).
Barazzoni, R., Short, K. R. & Nair, K. S. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J. Biol. Chem. 275, 3343–3347 (2000).
Ding, J. et al. Assessing mitochondrial DNA variation and copy number in lymphocytes of ~2,000 sardinians using tailored sequencing analysis tools. PLoS Genet. 11, e1005306 (2015).
Wachsmuth, M., Hübner, A., Li, M., Madea, B. & Stoneking, M. Age-related and heteroplasmy-related variation in human mtDNA copy number. PLoS Genet. 12, e1005939 (2016).
Skujina, I., McMahon, R., Lenis, V. P. E., Gkoutos, G. V. & Hegarty, M. Duplication of the mitochondrial control region is associated with increased longevity in birds. Aging 8, 1781–1789 (2016).
Garesse, R. & Vallejo, C. G. Animal mitochondrial biogenesis and function: a regulatory cross-talk between two genomes. Gene 263, 1–16 (2001).
Latorre-Pellicer, A. et al. Mitochondrial and nuclear DNA matching shapes metabolism and healthy ageing. Nature 535, 561–565 (2016).
Quiros, P. M., Goyal, A., Jha, P. & Auwerx, J. Analysis of mtDNA/nDNA ratio in mice. Curr. Protoc. Mouse Biol. 7, 47–54 (2017).
Rieckher, M., Markaki, M., Princz, A., Schumacher, B. & Tavernarakis, N. Maintenance of proteostasis by P body-mediated regulation of eIF4E availability during aging in Caenorhabditis elegans. Cell Rep. 25, 199–211 (2018).
Dillin, A. et al. Rates of behavior and aging specified by mitochondrial function during development. Science 298, 2398–2401 (2002).
van den Ecker, D. et al. Blue native electrophoresis to study mitochondrial complex I in C. elegans. Anal. Biochem. 407, 287–289 (2010).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Chen, C. et al. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol. Plant 13, 1194–1202 (2020).
Acknowledgements
We thank the members of the Tian laboratory, D. Chen and X. Zhan for discussion and technical assistance; Y. Qi for the polg-1 mutant strain; S. Zuryn for the germline mitochondrial marker strain; and S. Cai for the wild C. elegans strains. Several C. elegans strains used in this work were provided by CGC (supported by the NIH-Officer of Research Infrastructure Programs (P40 OD010440) and the Japanese National BioResource Project. This work was supported by the National Key R&D Program of China (2017YFA0506400, to Y.T.; and 2019YFA0508700, to W.Q.), the Strategic Priority Research Program of Chinese Academy of Sciences (XDB39000000, to Y.T.) and the National Natural Science Foundation of China (nos 31930023 and 31771333, to Y.T.; and no. 31922014, to W.Q.).
Author information
Authors and Affiliations
Contributions
Y.T. and Q.Z. conceived the study and designed the experiments. Q.Z., Q.W., Z.W. and J.Z. performed the C. elegans crosses and western blotting. Q.Z., W.Z. and Q.W. performed the mtDNA measurement, qPCR experiments, lifespan experiments and stress-resistance assay. Q.Z. performed BN-PAGE experiments, OCR measurement and data analysis. W.Z. performed the mtDNA measurement, qPCR experiments, western blotting in cell lines. X.L. performed the RNAi experiments and drug treatment. X.W. performed the ATP-level measurement. Y.G. and Y.L. performed plasmid and strain constructions. C.W. and W.Q. performed the RNA-seq analyses. Q.Z. prepared the original figures. Y.T. and Q.Z. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cell Biology thanks Doris Germain, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Representative fluorescence visualization of the transgenerational induction of the UPRmt across multiple generations.
a, Fluorescence visualization of the hsp-6p::gfp reporter in descendant animals generated from crosses between animals with neuronal mitochondrial stresses and wild-type animals. The schematic design of the experiment is presented in Fig. 1a. Worms with neuronal expression of Q40 or Wnt were crossed with WT males to generate the P0 animals with the expression of neuronal Q40 or Wnt. Then, P0 worms were allowed to produce F1 offspring through self-fertilization. 20%–30% of the F1 animals without expression of neuronal Q40 or Wnt still exhibited the strong induction of hsp-6p::gfp (indicated with white stars). We followed three independent lines with strong hsp-6p::gfp expression and one line that was not selected for the hsp-6p::gfp expression (randomly maintained) from each cross for over 50 generations. Worms with hsp-6p::gfp fluorescence over one-third of the intestine were considered to show the strong induction of the UPRmt. Scale bar, 250 μm. b, Percentage of worms showing the strong UPRmt induction in each generation as shown in a.
Extended Data Fig. 2 Disrupting mitochondrial function by RNAi or drugs did not trigger the transgenerational induction of UPRmt in their progeny.
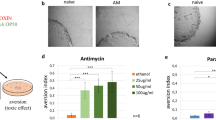
a, qRT-PCR analysis of exogenous expression of neuronal egl-20 in F1 animals with or without neuronal EGL-20 expression as described in Fig. 1d. n = 4 independent experiments. b, Fluorescence visualization of the hsp-6p::gfp reporter in F1 animals with or without neuronal cco-1 KD expression. Animals exhibiting the transgenerational induction of UPRmt generated from animals with neuronal cco-1 KD were referred to as ‘TGCK’. Data shown represent five independent experiments with similar results. c, The schematic diagram of the strategy used to induce the UPRmt in parental animals using RNAi or drug treatments and subsequently bleached these animals and transferred the progeny to the untreated plates. d-g, Fluorescence visualization of the hsp-6p::gfp reporter animals treated with different drugs and RNAi to perturb mitochondrial function, and their progeny recovered on OP50 plates as described. Data shown represent five independent experiments with similar results. h, Fluorescence visualization of the hsp-4p::gfp reporter in F1 animals with or without neuronal spliced xbp-1 generated from animals expressing neuronal xbp-1s. No transgenerational induction of UPRER was observed. Data shown represent five independent experiments with similar results. Scale bars, 250 μm. Data are the mean ± s.e.m.. Statistics source data are provided.
Extended Data Fig. 3 A schematic design of experiment to generate animals with the transgenerational induction of the UPRmt for mtDNA sequencing.
Worms used for the mtDNA sequencing are P0 animals with expression of neuronal Q40 or neuronal Wnt, and their F5 progeny with or without UPRmt induction. Data shown represent five independent experiments with similar results. Scale bar, 250 μm.
Extended Data Fig. 4 Animals with elevated levels of mtDNA causes mitochondrial proteostasis stress.
a, qRT-PCR analysis of nDNA-encoded and mtDNA-encoded OXPHOS genes in TGQ and WT animals. n = 3 independent experiments. b, qRT-PCR analysis of UPRmt target genes and polg-1, hmg-5, rpom-1, tfbm-1 in TGQ and WT animals. n = 3 independent experiments. c, Relative mtDNA levels of WT worms at different developmental stages. n = 7 independent experiments. d, e, mtDNA/nDNA ratios of TGW and WT animals. WT n = 102 worms, TGW n = 89 worms, WT D1 n = 22 worms, TGW D1 n = 22 worms, WT D5 n = 30 worms, TGW D5 n = 31 worms. f, Fold change in mtDNA/nDNA ratios of animals exhibited transgenerational UPRmt generated from animals with neuronal Q40 expression, or neuronal cco-1 KD. n = 4 independent experiments. g, Actual nDNA copies per worm of TGW, TGQ and WT animals. n = 5 independent experiments. Related to Fig. 2f. h, Fold change in germline mtDNA/nDNA ratios of animals expressing neuronal EGL-20 compared to WT. n = 5 independent experiments. i, Actual mtDNA copies per oocyte (position at ‘−1’, the one near to the spermatheca) of animals expressing neuronal EGL-20 compared to WT. n = 33 oocytes. j, qRT-PCR analysis of hsp-6 transcripts in the germline of TGW and WT animals. n = 3 independent experiments. k, Fluorescence visualization of the hsp-6p::gfp reporter in two groups of TGW animals according to their hsp-6p::gfp fluorescent intensities. Data shown represent three independent experiments with similar results. Scale bar, 250 μm. l, m, Representative immunoblots (l) and quantifications (m) of antibody sensitivity by western blot analyses of WT worms. n-p, Representative immunoblots (n) and quantifications (o, p) of mitochondrial OXPHOS proteins in TGQ and WT animals. n = 3 independent experiments. q, Western blot of purified mitochondria from TGQ and WT worms separated by Blue-native page gel using antibody against ATP5A (complex V). r, The oxygen consumption rates (r, n = 8 independent experiments) and the relative ATP levels (s, n = 3 independent experiments) of TGQ and WT animals. t-v, The mitochondria morphology in muscle (t), intestine (u), and oocytes (v) in TGW and WT worms. Scale bars, 10 μm. Data shown represent three independent experiments with similar results. Data are the mean ± s.e.m., ***P < 0.001, **P < 0.01, *P < 0.05, ns denotes P > 0.05, P values were determined using two-way ANOVA (a, b), one-way ANOVA (f, g) and two-sided Student’s t-test (d, e, h, i, j, o, p, r, s). The exact P values are provided in Source Data Extended Data Fig. 4. Statistics source data and unprocessed western blots are provided.
Extended Data Fig. 5 UPRmt machinery is not required for the transmission of increased mtDNA levels across generations.
a, b, Fluorescence visualization of the hsp-6p::gfp reporter (a) and fold change in relative mtDNA levels (b) in TGQ, TGQ;atfs-1(-/-) animals. n = 4 independent experiments. c-e, Representative immunoblots (c) and quantifications (d, e) of mitochondrial OXPHOS proteins in atfs-1 and TGQ; atfs-1 animals. n = 3 independent experiments. f, g, Fluorescence visualization (f) and quantifications (g) of the hsp-6p::gfp reporter in TGW, TGW;lin-65(-/-) and TGW;lin-65(+/+) animals. TGW n = 34 worms, TGW;lin-65(-/-) n = 41 worms, TGW;lin-65(+/+) n = 30 worms. h, Fold change in mtDNA/nDNA ratios of animals shown in (f). n = 3 independent experiments. i, Relative mtDNA levels in F1 animals generated from the cross between neuronal EGL-20; atfs-1(gk3094) hermaphrodites and atfs-1(gk3094) males. The cross strategy was described in Fig. 1a. n = 3 independent experiments. Scale bars, 250 μm. Data are the mean ± s.e.m., ***P < 0.001, **P < 0.01, *P < 0.05, ns denotes P > 0.05, P values were determined using one-way ANOVA (b, g, h, i) and two-sided Student’s t-test (d, e). The exact P values are provided in Source Data Extended Data Fig. 5. Statistics source data and unprocessed western blots are provided.
Extended Data Fig. 6 Wnt signaling is involved in the transmission of increased mtDNA levels across generations and is conserved to regulate mtDNA biogenesis.
a, qRT-PCR analysis of egl-20 in TGW, TGQ and WT animals. n = 8 independent experiments. b, c, Fluorescence visualization of the hsp-6p::gfp(b) and fold change in relative mtDNA levels (c) in TGQ, TGQ;egl-20(-/-) animals. n = 4 independent experiments. d-f, Representative immunoblots (d) and quantifications (e, f) of mitochondrial OXPHOS proteins in egl-20(-/-) and TGQ;egl-20(-/-) animals. n = 3 independent experiments. g, h, Fluorescence visualization (g) and quantifications (h) of the hsp-6p::gfp reporter in TGW (n = 32 worms), TGW;vps-35(-/-) (n = 30 worms) and TGW;vps-35(+/+) (n = 30 worms) animals. i, Fold change in mtDNA/nDNA ratios of animals shown in (g). n = 4 independent experiments. j, k, Fluorescence visualization (j) and quantifications (k) of the hsp-6p::gfp reporter in TGW (n = 31 worms), TGW;mig-14(-/-) (n = 30 worms) and TGW;mig-14(+/+) (n = 31 worms). l, Fold change in mtDNA/nDNA ratios of animals shown in (j). n = 4 independent experiments. m, n, Fluorescence visualization (m) and quantifications (n) of the hsp-6p::gfp reporter in TGW animals in different mutant background. WT n = 35 worms, cwn-1 n = 35 worms, cwn-2 n = 29 worms, lin-44 n = 34 worms, lin-17 n = 29 worms, mig-1 n = 30 worms. o, Fold change in mtDNA/nDNA ratios of animals shown in m. n = 3 independent experiments. p, Fluorescence visualization of the hsp-6p::gfp reporter in TGW animals treated with EV or bar-1 RNAi. Data shown represent three independent experiments with similar results. q, r, Fold change in mtDNA/nDNA ratios of TGW and WT animals treated with EV or bar-1 RNAi that since oogenesis (q, n = 6 independent experiments) and zygotically development (r, n = 5 independent experiments). s, t, Wnt target gene axin2 transcripts (s) and β-catenin protein level (t) in HEK293T cells treated with different concentrations of CHIR-99021 for 6 h. n = 3 independent experiments. u, v, Wnt target gene axin2 transcripts (u) and β-catenin protein level (v) in HEK293T cells treated with 10 µM CHIR-99021 at different time points. n = 4 independent experiments. w, x, qRT-PCR analysis of β-catenin or Myc in HEK293T cells treated with β-catenin or Myc siRNA. n = 4 independent experiments. Scale bars, 250 μm. Data are the mean ± s.e.m., ***P < 0.001, **P < 0.01, *P < 0.05, ns denotes P > 0.05, P values were determined using two-way ANOVA (q, r), one-way ANOVA (a, c, h, I, k, l, n, o, s, u) and two-sided Student’s t-test (e, f, w, x). The exact P values are provided in Source Data Extended Data Fig. 6. Statistics source data and unprocessed western blots are provided.
Extended Data Fig. 7 Distribution of SNPs and indels of genome for ED3011 and KR314.
a, Circos-plot of mitochondrial genome features of ED3011 and KR314. The circles from the inside going in represent the C. elegans mitochondrial genome, nucleotide variations of KR314 and ED3011 (as the black line indicated) compared to N2. The circles on the outside represent the gene names on the mtDNA. b, Circos-plot of nuclear genome features of ED3011 and KR314. Segmented circles on the inside represent the six C. elegans chromosomes. Track 1, 2, 3, 4 are the histograms represent the numbers of variations per 10 kb (outward pointing bars = higher density), Track 1 shows the SNPs of ED3011. Track 2 shows the SNPs of KR314.Track 3 represents the indels of ED3011. Track 4 represents the indels of KR314. Track 5 is the heatmap depicts the density of CDS (dark red = higher density) on C. elegans chromosomes. Circos graph were generated with the basic information of C. elegans genome WBcel235 (BioProject PRJNA13758) using TBtools61. See Supplementary Table 5 for the list of genetic variations identified in wild C. elegans strain ED3011 and KR314.
Extended Data Fig. 8 The transgenerational induction of the UPRmt provides benefits to offspring under stress conditions.
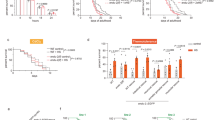
a-d, Lifespan analysis of TGW and TGQ animals compared to WT animals. e-h, PA14 slow killing assay of TGW and TGQ animals compared to WT animals. i, Survival rates of day 1 adult WT and TGQ animals after paraquat treatment. n = 4 independent experiments. j, Survival rates of D1 adult WT and TGQ animals after heat shock treatment. n = 5 independent experiments. k, Lifespan analysis of N2 and other wild C. elegans strains ED3011 and KR314. l-o, Lifespan analysis of TGQ and TGW animals in polg-1(tm2685) (l), fzo-1(tm1133) (m), atfs-1(gk3094) (n) and egl-20(n585) (o) background. Data are the mean ± s.e.m., ***P < 0.001, **P < 0.01, P values were determined using two-sided Student’s t-test (i, j). The exact P values are provided in Source Data Extended Data Fig. 8. Statistics source data are provided.
Supplementary information
Supplementary Table 1
Primer sequences used in this study.
Supplementary Table 2
Strain list used in this study.
Supplementary Table 3
Summary of mtDNA variations identified in TGQ and TGW animals.
Supplementary Table 4
Differentially expressed genes in TGQ worms as identified by RNA-seq analyses.
Supplementary Table 5
Summary of genetic variations identified in wild C. elegans strains ED3011, KR314 and CB4856.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed western blots.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 3
Unprocessed western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 8
Statistical source data.
Rights and permissions
About this article
Cite this article
Zhang, Q., Wang, Z., Zhang, W. et al. The memory of neuronal mitochondrial stress is inherited transgenerationally via elevated mitochondrial DNA levels. Nat Cell Biol 23, 870–880 (2021). https://doi.org/10.1038/s41556-021-00724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-021-00724-8
This article is cited by
-
Mitochondrial stress: a key role of neuroinflammation in stroke
Journal of Neuroinflammation (2024)
-
Emerging roles of mitochondrial functions and epigenetic changes in the modulation of stem cell fate
Cellular and Molecular Life Sciences (2024)
-
Maternal aging increases offspring adult body size via transmission of donut-shaped mitochondria
Cell Research (2023)
-
Histone H3K4me3 modification is a transgenerational epigenetic signal for lipid metabolism in Caenorhabditis elegans
Nature Communications (2022)
-
Pleiotropic effects of mitochondria in aging
Nature Aging (2022)