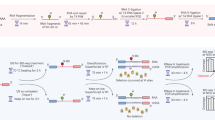
Abstract
Although high-throughput RNA sequencing (RNA-seq) has greatly advanced small non-coding RNA (sncRNA) discovery, the currently widely used complementary DNA library construction protocol generates biased sequencing results. This is partially due to RNA modifications that interfere with adapter ligation and reverse transcription processes, which prevent the detection of sncRNAs bearing these modifications. Here, we present PANDORA-seq (panoramic RNA display by overcoming RNA modification aborted sequencing), employing a combinatorial enzymatic treatment to remove key RNA modifications that block adapter ligation and reverse transcription. PANDORA-seq identified abundant modified sncRNAs—mostly transfer RNA-derived small RNAs (tsRNAs) and ribosomal RNA-derived small RNAs (rsRNAs)—that were previously undetected, exhibiting tissue-specific expression across mouse brain, liver, spleen and sperm, as well as cell-specific expression across embryonic stem cells (ESCs) and HeLa cells. Using PANDORA-seq, we revealed unprecedented landscapes of microRNA, tsRNA and rsRNA dynamics during the generation of induced pluripotent stem cells. Importantly, tsRNAs and rsRNAs that are downregulated during somatic cell reprogramming impact cellular translation in ESCs, suggesting a role in lineage differentiation.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
RNA-seq datasets have been deposited in the Gene Expression Omnibus under the accession code GSE144666. LC-MS/MS data have been deposited in Figshare (https://figshare.com/articles/dataset/_/14033003). All other data supporting the findings of this study are available from the corresponding authors upon reasonable request. Source data are provided with this paper.
Code availability
The sncRNA annotation pipeline SPORTS1.1 is available from GitHub (https://github.com/junchaoshi/sports1.1). The scripts used for data processing and statistical analysis were written in Perl or R and are available upon reasonable request.
Change history
29 April 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41556-021-00687-w
References
Bartel, D. P. Metazoan microRNAs. Cell 173, 20–51 (2018).
Honda, S. et al. Sex hormone-dependent tRNA halves enhance cell proliferation in breast and prostate cancers. Proc. Natl Acad. Sci. USA 112, E3816–E3825 (2015).
Cozen, A. E. et al. ARM-seq: AlkB-facilitated RNA methylation sequencing reveals a complex landscape of modified tRNA fragments. Nat. Methods 12, 879–884 (2015).
Zheng, G. et al. Efficient and quantitative high-throughput tRNA sequencing. Nat. Methods 12, 835–837 (2015).
Dai, Q., Zheng, G., Schwartz, M. H., Clark, W. C. & Pan, T. Selective enzymatic demethylation of N2,N2-dimethylguanosine in RNA and its application in high-throughput tRNA sequencing. Angew. Chem. Int. Ed. Engl. 56, 5017–5020 (2017).
Zhang, X., Cozen, A. E., Liu, Y., Chen, Q. & Lowe, T. M. Small RNA modifications: integral to function and disease. Trends Mol. Med. 22, 1025–1034 (2016).
Chen, Q., Yan, W. & Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 17, 733–743 (2016).
Sergiev, P. V., Aleksashin, N. A., Chugunova, A. A., Polikanov, Y. S. & Dontsova, O. A. Structural and evolutionary insights into ribosomal RNA methylation. Nat. Chem. Biol. 14, 226–235 (2018).
Phizicky, E. M. & Hopper, A. K. tRNA biology charges to the front. Genes Dev. 24, 1832–1860 (2010).
Schimmel, P. The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis. Nat. Rev. Mol. Cell Biol. 19, 45–58 (2018).
Akiyama, Y. et al. Multiple ribonuclease A family members cleave transfer RNAs in response to stress. Preprint at biorxiv https://doi.org/10.1101/811174 (2019).
Shigematsu, M., Kawamura, T. & Kirino, Y. Generation of 2′,3′-cyclic phosphate-containing RNAs as a hidden layer of the transcriptome. Front. Genet. 9, 562 (2018).
Akat, K. M. et al. Detection of circulating extracellular mRNAs by modified small-RNA-sequencing analysis. JCI Insight 5, e127317 (2019).
Giraldez, M. D. et al. Phospho-RNA-seq: a modified small RNA-seq method that reveals circulating mRNA and lncRNA fragments as potential biomarkers in human plasma. EMBO J. 38, e101695 (2019).
Shi, J., Zhang, Y., Zhou, T. & Chen, Q. tsRNAs: the Swiss Army knife for translational regulation. Trends Biochem. Sci. 44, 185–189 (2019).
Su, Z., Wilson, B., Kumar, P. & Dutta, A. Noncanonical roles of tRNAs: tRNA fragments and beyond. Annu. Rev. Genet. 54, 47–69 (2020).
Zhang, Y. et al. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat. Cell Biol. 20, 535–540 (2018).
Natt, D. et al. Human sperm displays rapid responses to diet. PLoS Biol. 17, e3000559 (2019).
Gu, W. et al. Peripheral blood non-canonical small non-coding RNAs as novel biomarkers in lung cancer. Mol. Cancer 19, 159 (2020).
Shi, J., Ko, E. A., Sanders, K. M., Chen, Q. & Zhou, T. SPORTS1.0: a tool for annotating and profiling non-coding RNAs optimized for rRNA- and tRNA-derived small RNAs. Genom. Proteom. Bioinf. 16, 144–151 (2018).
Trewick, S. C., Henshaw, T. F., Hausinger, R. P., Lindahl, T. & Sedgwick, B. Oxidative demethylation by Escherichia coli AlkB directly reverts DNA base damage. Nature 419, 174–178 (2002).
Chen, Q. et al. Sperm tsRNAs contribute to intergenerational inheritance of an acquired metabolic disorder. Science 351, 397–400 (2016).
Pan, T. Modifications and functional genomics of human transfer RNA. Cell Res. 28, 395–404 (2018).
Guo, G. et al. Epigenetic resetting of human pluripotency. Development 144, 2748–2763 (2017).
Cheloufi, S. et al. The histone chaperone CAF-1 safeguards somatic cell identity. Nature 528, 218–224 (2015).
Peng, H. et al. A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Res. 22, 1609–1612 (2012).
Chu, C. et al. A sequence of 28S rRNA-derived small RNAs is enriched in mature sperm and various somatic tissues and possibly associates with inflammation. J. Mol. Cell. Biol. 9, 256–259 (2017).
Valdmanis, P. N. et al. RNA interference-induced hepatotoxicity results from loss of the first synthesized isoform of microRNA-122 in mice. Nat. Med. 22, 557–562 (2016).
Zhang, P. et al. piRBase: a web resource assisting piRNA functional study. Database (Oxf.) 2014, bau110 (2014).
Sai Lakshmi, S. & Agrawal, S. piRNABank: a web resource on classified and clustered PIWI-interacting RNAs. Nucleic Acids Res. 36, D173–D177 (2008).
Suzuki, T. & Suzuki, T. A complete landscape of post-transcriptional modifications in mammalian mitochondrial tRNAs. Nucleic Acids Res. 42, 7346–7357 (2014).
Hizir, Z., Bottini, S., Grandjean, V., Trabucchi, M. & Repetto, E. RNY (YRNA)-derived small RNAs regulate cell death and inflammation in monocytes/macrophages. Cell Death Dis. 8, e2530 (2017).
Viswanathan, S. R., Daley, G. Q. & Gregory, R. I. Selective blockade of microRNA processing by Lin28. Science 320, 97–100 (2008).
Krishna, S. et al. Dynamic expression of tRNA-derived small RNAs define cellular states. EMBO Rep. 20, e47789 (2019).
Zhang, J. et al. Metabolism in pluripotent stem cells and early mammalian development. Cell Metab. 27, 332–338 (2018).
Chau, K. F. et al. Downregulation of ribosome biogenesis during early forebrain development. eLife 7, e36998 (2018).
Yang, X. et al. Single sample expression-anchored mechanisms predict survival in head and neck cancer. PLoS Comput. Biol. 8, e1002350 (2012).
Genuth, N. R. & Barna, M. The discovery of ribosome heterogeneity and its implications for gene regulation and organismal life. Mol. Cell 71, 364–374 (2018).
Li, D. & Wang, J. Ribosome heterogeneity in stem cells and development. J. Cell Biol. 219, e202001108 (2020).
Ozata, D. M., Gainetdinov, I., Zoch, A., O’Carroll, D. & Zamore, P. D. PIWI-interacting RNAs: small RNAs with big functions. Nat. Rev. Genet. 20, 89–108 (2019).
Lambert, M., Benmoussa, A. & Provost, P. Small non-coding RNAs derived from eukaryotic ribosomal RNA. Noncoding RNA 5, 16 (2019).
Wei, H. et al. Profiling and identification of small rDNA-derived RNAs and their potential biological functions. PLoS ONE 8, e56842 (2013).
Thompson, D. M., Lu, C., Green, P. J. & Parker, R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA 14, 2095–2103 (2008).
Liao, J. Y. et al. Both endo-siRNAs and tRNA-derived small RNAs are involved in the differentiation of primitive eukaryote Giardia lamblia. Proc. Natl Acad. Sci. USA 111, 14159–14164 (2014).
Lambertz, U. et al. Small RNAs derived from tRNAs and rRNAs are highly enriched in exosomes from both Old and New World Leishmania providing evidence for conserved exosomal RNA packaging. BMC Genomics 16, 151 (2015).
Garcia-Silva, M. R. et al. Extracellular vesicles shed by Trypanosoma cruzi are linked to small RNA pathways, life cycle regulation, and susceptibility to infection of mammalian cells. Parasitol. Res. 113, 285–304 (2014).
Fricker, R. et al. A tRNA half modulates translation as stress response in Trypanosoma brucei. Nat. Commun. 10, 118 (2019).
Zinskie, J. A. et al. Iron-dependent cleavage of ribosomal RNA during oxidative stress in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 293, 14237–14248 (2018).
Yamasaki, S., Ivanov, P., Hu, G. F. & Anderson, P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 185, 35–42 (2009).
Lee, S. R. & Collins, K. Starvation-induced cleavage of the tRNA anticodon loop in Tetrahymena thermophila. J. Biol. Chem. 280, 42744–42749 (2005).
Andersen, K. L. & Collins, K. Several RNase T2 enzymes function in induced tRNA and rRNA turnover in the ciliate Tetrahymena. Mol. Biol. Cell 23, 36–44 (2012).
Kuscu, C. et al. tRNA fragments (tRFs) guide Ago to regulate gene expression post-transcriptionally in a Dicer-independent manner. RNA 24, 1093–1105 (2018).
Luo, S. et al. Drosophila tsRNAs preferentially suppress general translation machinery via antisense pairing and participate in cellular starvation response. Nucleic Acids Res. 46, 5250–5268 (2018).
Kim, H. K. et al. A transfer-RNA-derived small RNA regulates ribosome biogenesis. Nature 552, 57–62 (2017).
Gebetsberger, J., Wyss, L., Mleczko, A. M., Reuther, J. & Polacek, N. A tRNA-derived fragment competes with mRNA for ribosome binding and regulates translation during stress. RNA Biol. 14, 1364–1373 (2017).
Schorn, A. J., Gutbrod, M. J., LeBlanc, C. & Martienssen, R. LTR-retrotransposon control by tRNA-derived small RNAs. Cell 170, 61–71.e11 (2017).
Martinez, G., Choudury, S. G. & Slotkin, R. K. tRNA-derived small RNAs target transposable element transcripts. Nucleic Acids Res. 45, 5142–5152 (2017).
Sarker, G. et al. Maternal overnutrition programs hedonic and metabolic phenotypes across generations through sperm tsRNAs. Proc. Natl Acad. Sci. USA 116, 10547–10556 (2019).
Sharma, U. et al. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 351, 391–396 (2016).
Zhang, Y., Shi, J., Rassoulzadegan, M., Tuorto, F. & Chen, Q. Sperm RNA code programmes the metabolic health of offspring. Nat. Rev. Endocrinol. 15, 489–498 (2019).
Ren, B., Wang, X., Duan, J. & Ma, J. Rhizobial tRNA-derived small RNAs are signal molecules regulating plant nodulation. Science 365, 919–922 (2019).
Lewis, C. J., Pan, T. & Kalsotra, A. RNA modifications and structures cooperate to guide RNA–protein interactions. Nat. Rev. Mol. Cell Biol. 18, 202–210 (2017).
Frye, M., Harada, B. T., Behm, M. & He, C. RNA modifications modulate gene expression during development. Science 361, 1346–1349 (2018).
Raabe, C. A., Tang, T. H., Brosius, J. & Rozhdestvensky, T. S. Biases in small RNA deep sequencing data. Nucleic Acids Res. 42, 1414–1426 (2014).
Wei, F. Y. et al. Cdk5rap1-mediated 2-methylthio modification of mitochondrial tRNAs governs protein translation and contributes to myopathy in mice and humans. Cell Metab. 21, 428–442 (2015).
Li, L., Dai, H., Nguyen, A. P. & Gu, W. A convenient strategy to clone modified/unmodified small RNA and mRNA for high throughput sequencing. RNA 16, 218–222 (2019).
Stadtfeld, M., Maherali, N., Borkent, M. & Hochedlinger, K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat. Methods 7, 53–55 (2010).
Behringer, R., Gertsenstein, M., Nagy, K. V. & Nagy, A. Differentiating mouse embryonic stem cells into embryoid bodies by hanging-drop cultures. Cold Spring Harb. Protoc. https://doi.org/10.1101/pdb.prot092429 (2016).
Schaniel, C. et al. Delivery of short hairpin RNAs—triggers of gene silencing—into mouse embryonic stem cells. Nat. Methods 3, 397–400 (2006).
Kozomara, A. & Griffiths-Jones, S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68–D73 (2014).
Chan, P. P. & Lowe, T. M. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 44, D184–D189 (2016).
Juhling, F. et al. tRNAdb 2009: compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 37, D159–D162 (2009).
Yates, A. et al. Ensembl 2016. Nucleic Acids Res. 44, D710–D716 (2016).
Nawrocki, E. P. et al. Rfam 12.0: updates to the RNA families database. Nucleic Acids Res. 43, D130–D137 (2015).
Wang, L., Feng, Z., Wang, X., Wang, X. & Zhang, X. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010).
Lorenz, R. et al. ViennaRNA Package 2.0. Algorithms Mol. Biol. 6, 26 (2011).
Bray, N. L., Pimentel, H., Melsted, P. & Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 34, 525–527 (2016).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Acknowledgements
We thank T. Lowe at the University of California, Santa Cruz for early discussion on the project, and Z. Li from the Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences for assistance with operating the mass spectrometer. This work is in part supported by MOST (2019YFA0802600 to Ying Zhang (Chinese Academy of Sciences) and Yunfang Zhang; 2018YFC1004500 to Ying Zhang (Chinese Academy of Sciences) and M.Y.), startup funds from the University of California, Riverside (to Q.C. and S.C.) and the NIH (R01HD092431 to Q.C.; R01ES032024 to Q.C. and T.Z.; P50HD098593 to T.Z. and Q.C.; R35GM128854 to L. Zhao). This work includes data generated at the University of California, San Diego IGM Genomics Center funded by the NIH (P30DK063491, P30CA023100 and P30DK120515). Q.Z. is funded by the NSFC (31630037). Ying Zhang (University of California, Riverside) is funded by a State Scholarships Fund (201908500039). Yunfang Zhang is funded by the NSFC (82022029) and the Natural Science Foundation of Chongqing (cstc2019jcyjjqX0010). M.Y. is funded by the NSFC (31670830) and is a fellow of the Innovative Research Team of High-Level Local Universities in Shanghai. M.S. is funded by an Advanced EMBO fellowship. K.M. is funded by a BBSRC scholarship. Work in the laboratory of M.Z.-G. is funded by the Wellcome Trust (207415/Z/17/Z), ERC (669198) and Open Philanthropy. R.F. is supported by UC Riverside’s Eugene Cota-Robles Fellowship.
Author information
Authors and Affiliations
Contributions
Q.C., T.Z. and J.S. designed the project. Yunfang Zhang, D.T. and J.S. developed and optimized the enzymatic treatment protocol for PANDORA-seq. J.S., T.Z., Ying Zhang (Chinese Academy of Sciences) and Q.C. designed and developed the scope of data analyses. S.C., J.M. and R.F. generated iPSCs from MEFs and contributed to related analyses. X.Z. and R.F. performed the functional assays of mESCs under the supervision of S.C. and Q.C. J.S. and T.Z. developed the computational tools and analysed all of the datasets with input from Ying Zhang (Chinese Academy of Sciences) and Q.C. X.Z. and Ying Zhang (University of California, Riverside) developed and performed northern blot analyses for tissues/cells with help from D.T. and S.L. Yunfang Zhang tested and validated T4PNK’s effect in improving adapter ligation. M.Y. and X.Z. contributed to the LC-MS/MS RNA modification analyses with the help from Y.W. M.Y. designed and generated the AlkB plasmid and generated the AlkB enzyme with help from W.Z., Q.Z. and L. Zhao. L. Zhang and Y.Q. collected mature sperm samples under the supervision of Ying Zhang (Chinese Academy of Sciences). M.S., K.M. and M.Z.-G. performed experiments to contribute mESCs, primed hESCs and naive hESCs for analyses. B.R.C. contributed to data interpretation in regard to piRNAs and rsRNAs and the Discussion section, with input from D.T.C., J.G. and E.R.J. X.C. contributed to data interpretation in regard to miRNA and miRBase. P.S., X.-l.Y. and B.K. contributed to data interpretation of mitochondrial tsRNAs and discussed the evolutionary aspects. L. Zhao, C.Z., W.G., D.T.C., J.G. and E.R.J. contributed to the interpretation and discussion of data. Q.C. T.Z., Ying Zhang (Chinese Academy of Sciences) and J.S. wrote the main manuscript and integrated input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Cell Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Reads summary and length distributions of different sncRNA category under Traditional RNA-seq, AlkB-facilitated RNA-seq, T4PNK-facilitated RNA-seq, and PANDORA-seq.
Showing Reads summary and length distributions of different sncRNA category in six tissue/cell types that are not shown in Fig. 3 because of space limitation. (a-c) Cells during mouse somatic cell reprogramming to iPSC: (a) MEFs (day 0), (b) intermediates (day 3), (c) iPSCs; (d) mouse spleen, (e) primed human embryonic stem cells (hESCs-primed), and (f) naïve human embryonic stem cells (hESCs-naïve) (g-l) the relative tsRNA/miRNA ratio under different protocols. for g,h,I,k, mean ± SEM, n=3 biologically independent samples in each bar; for j,l, n=2 biologically independent samples in each bar; different letters above bars indicate statistical difference, P < 0.05; same letters indicate P ≥ 0.05 (two-sided, one-way ANOVA, uncorrected Fisher’s LSD test). Statistical source data and the precise P values are provided in Source Data Extended Data Fig. 1.
Extended Data Fig. 2 Evaluation of Northern blot probe efficiency on synthesized targets (that is, rsRNA-28S-1, 5’tsRNAGlu, let-7i, mir-122, mir-21).
The Northern blot probes used for each target are the same as used in main Fig. 2g-i. a, each synthetic sncRNAs are individually loaded on PAGE followed by Northern blots analyses. b, the five synthetic sncRNAs were mixed together with the amount tested in (a) and then equally separated and loaded on PAGE followed by Northern blots analyses. The relative efficiency of each NB probe can be shown: the probe efficiency between let-7i, tsRNAGlu and rsRNA-28 are similar; the probe for mir-122 is highest, while the probe for mir-21 has the lowest efficiency. Similar results were obtained in 3 independent experiments. The unprocessed blots are provided in Source Data Extended Data Fig. 2.
Extended Data Fig. 3 Annotation of mouse piRNA in non-germ cell tissue/cell types is not stable when 1–3 mismatches are allowed.
When 1–3 mismatches are allowed for sncRNAs matching, the piRNA annotation rate (but not other sncRNAs types) show significant decrease in mouse tissue/cell types (a) mouse brain, (b) mouse liver, (c) mouse spleen, (d) mouse embryonic stem cells, (e) mouse mature sperm, (f) mouse mature sperm heads, (g) mouse MEFs (day 0), (h) mouse intermediate cells (day 3), (i) mouse iPSCs. Very few piRNAs were annotated for human cell lines (j) human HeLa cells, (k) human hESCs-primed, and (l) human hESCs-naïve. These data suggest the annotated piRNAs in non-germ cell tissue/cell types could be due to database quality issue and their true identity awaits to be verified.
Extended Data Fig. 4 Scattered plot comparison of profile changes in tsRNAs and rsRNAs compared to miRNAs under different treatment protocol.
Scattered plot comparison of profile changes in tsRNAs (red dots) and rsRNAs (blue dots) compared to miRNAs (gray dots) under AlkB vs traditional, T4PNK vs traditional and PANDORA-seq vs traditional in (a) mouse brain, (b) mouse liver, (c) mouse spleen, (d) mouse mature sperm, (e) mouse MEFs (day 0), (f) mouse intermediate cells (day 3), (g) mouse iPSCs, (h) human HeLa cells, (i) human hESCs-primed, (j) mouse mature sperm heads, and (k) human hESCs-naïve.
Extended Data Fig. 5 The tsRNA responses to AlkB, T4PNK and PANDORA-seq in regard to different tsRNA origin (5’tsRNA, 3’tsRNA, 3’tsRNA with CCA end, and internal tsRNAs).
a, mouse brain, (b) mouse liver, (c) mouse spleen, (d) mouse mature sperm, (e) mouse mature sperm heads, (f) mouse MEFs (day 0), (g) mouse intermediate cells (day 3), (h) mouse iPSCs, (i) human HeLa cells, (j) human hESCs-primed, and (k) human hESCs-naïve. For a-b,d-j, data are plotted as mean ± SEM (n=3 biologically independent samples in each bar); for c,k, n=2 biologically independent samples in each bar. Different letters above bars indicate statistical difference, P < 0.05; same letters indicate P ≥ 0.05 (two-sided, one-way ANOVA, uncorrected Fisher’s LSD test). Statistical source data and the precise P values are provided in Source Data Extended Data Fig. 5.
Extended Data Fig. 6 Overall length mapping of tsRNA reads in genomic and mitochondrial tRNA under different RNA-seq protocol.
Overall mapping of all tsRNAs on a tRNA length scale revealed the preferential loci from which tsRNAs are derived from the mature full tRNA under traditional protocol and different enzymatic treatments. a, mouse brain, (b) mouse liver, (c) mouse spleen, (d) mouse mature sperm, (e) mouse MEFs (day 0), (f) mouse intermediate cells (day 3), (g) mouse iPSCs, (h) human HeLa cells, (i) human hESCs-primed, (j) mouse mature sperm heads, and (k) human hESCs-naïve. Mapping plots are presented as mean ± SEM.
Extended Data Fig. 7 The miRNAs that showing sensitive response to PANDORA-seq are in fact rsRNAs.
Previously annotated miRNAs from miRbase that showing upregulation under PANDORA-seq could also annotated to rsRNAs (with one mismatch tolerance), as shown in (a) mouse brain, (b) mouse liver, (c) mouse spleen, (d) mouse mature sperm, (e) mouse mature sperm heads, (f) mouse MEFs (day 0), (g) mouse intermediate cells (day 3), (h) mouse iPSCs, (i) human HeLa cells, and (j) human hESCs-naïve.
Extended Data Fig. 8 The pairwise comparison matrices showing the differential expression pattern of rsRNAs under different RNA-seq protocol across tissues and cells.
a, Pairwise comparison matrices for six mouse tissue/cell types, including 5S rRNA, 5.8S rRNA, mitochondrial 12S rRNA, mitochondrial 16S rRNA, 28S rRNA and 45S rRNA. Color bar: from blue (more similar) to red (more different). b, Pairwise comparison matrices for three human cell types, including 5S rRNA, 5.8S rRNA, mitochondrial 12S rRNA, mitochondrial 16S rRNA, 28S rRNA and 45S rRNA. Color bar: from blue (more similar) to red (more different). c, Pairwise comparison matrices for during mouse iPSC reprogramming, including 5S rRNA, 5.8S rRNA, mitochondrial 12S rRNA, mitochondrial 16S rRNA, 18S rRNA, 28S rRNA and 45S rRNA. Color bar: from blue (more similar) to red (more different).
Extended Data Fig. 9 Northern blot analyses of tsRNA/rsRNA (that is, tsRNAAla, tsRNAArg, tsRNAGlu, tsRNAHis, tsRNALys and rsRNA-28S-1) changes during mESC to EB differentiation.
a, mESC vs Day6 EB; (b) mESC vs Day10 EB. Red arrowhead: tsRNAs; Blue arrowhead: rsRNAs. Similar results were obtained in 3 independent experiments for rsRNA-28S-1; and in 2 independent experiments for tsRNAAla, tsRNAArg, tsRNAGlu, tsRNAHis, and tsRNALys. The unprocessed blots are provided in Source Data Extended Data Fig. 9.
Extended Data Fig. 10 Expression heatmap of the differentially expressed genes from representative GOBP terms in Day6 and Enriched GOBP terms of differential expressed genes in Day3 EBs after tsRNA/rsRNA transfection.
a,b,c,d, Expression heatmap of the differentially expressed genes from the representative GOBP terms in Day3 EBs from Fig. 6b,c: (a) Neurological development; (b) Muscle/heart development; (c) Oxidative phosphorylation; (d) Translation/ribosome. Venn-diagram beneath each heatmap shows the numbers of overlapped dysregulated genes under different tsRNA/rsRNA transfection. e, Top-ranked upregulated GOBP terms in Day3 EBs after each tsRNA/rsRNA transfection compared to control. f, Top-ranked downregulated GOBP terms in Day3 EBs after each tsRNA/rsRNA transfection compared to control.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3.
Supplementary Tables
Supplementary Table 1. RNA-seq read summaries and differentially expressed sncRNAs by pairwise comparison between individual RNA-seq protocols. Supplementary Table 2. Alternative annotation for miRNA fragments based on miRBase among mouse and human tissues/cells. Supplementary Table 3. Statistics of probes targeting small RNA expression between MEFs and iPSCs under traditional treatment. Supplementary Table 4. List of differentially expressed genes in day 1, 3 and 6 embryoid bodies after tsRNA/rsRNA transfection. Supplementary Table 5. Gene set scores for GOBP terms.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 1
Unprocessed gels.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 2
Unprocessed gels.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed gels.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Unprocessed gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed gels.
Rights and permissions
About this article
Cite this article
Shi, J., Zhang, Y., Tan, D. et al. PANDORA-seq expands the repertoire of regulatory small RNAs by overcoming RNA modifications. Nat Cell Biol 23, 424–436 (2021). https://doi.org/10.1038/s41556-021-00652-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41556-021-00652-7
This article is cited by
-
tRNA-derived small RNAs in human cancers: roles, mechanisms, and clinical application
Molecular Cancer (2024)
-
Terminal modifications independent cell-free RNA sequencing enables sensitive early cancer detection and classification
Nature Communications (2024)
-
tRNA therapeutics for genetic diseases
Nature Reviews Drug Discovery (2024)
-
Small RNA structural biochemistry in a post-sequencing era
Nature Protocols (2024)
-
Future in the past: paternal reprogramming of offspring phenotype and the epigenetic mechanisms
Archives of Toxicology (2024)